 |
Name: AGRYLIN® (anagrelide hydrochloride)
Dosage Form: 0.5 mg and 1 mg capsules for oral administration
Active Ingredient: AGRYLIN® Capsules contain either 0.5 mg or 1 mg of anagrelide base (as anagrelide hydrochloride).
Inactive Ingredients: Povidone USP, Anhydrous Lactose NF, Lactose Monohydrate NF, Microcrystalline Cellulose NF, Crospovidone NF, Magnesium Stearate NF.
Pharmacological Classification: Platelet-reducing agent.
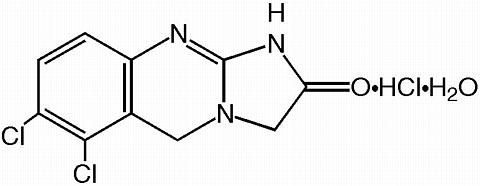
Chemical Name: 6,7-dichloro-1,5-dihydroimidazo[2,1-b]quinazolin-2(3H)-one monohydrochloride monohydrate.
Molecular formula: C 10 H 7 Cl 2 N 3 O·HCl·H 2 O
 |
Appearance: Off-white powder.
Solubility: Water ............................. Very slightly soluble
Dimethyl Sulfoxide .............. Sparingly soluble
Dimethylformamide ..............Sparingly soluble
The mechanism by which anagrelide reduces blood platelet count is still under investigation. Studies in patients support a hypothesis of dose-related reduction in platelet production resulting from a decrease in megakaryocyte hypermaturation. In blood withdrawn from normal volunteers treated with anagrelide, a disruption was found in the postmitotic phase of megakaryocyte development and a reduction in megakaryocyte size and ploidy. At therapeutic doses, anagrelide does not produce significant changes in white cell counts or coagulation parameters, and may have a small, but clinically insignificant effect on red cell parameters. Platelet aggregation is inhibited in people at doses higher than those required to reduce platelet count. Anagrelide inhibits cyclic AMP phosphodiesterase, as well as ADP- and collagen-induced platelet aggregation.
Following oral administration of 14 C-anagrelide in people, more than 70% of radioactivity was recovered in urine. Based on limited data, there appears to be a trend toward dose linearity between doses of 0.5 mg and 2.0 mg. At fasting and at a dose of 0.5 mg of anagrelide, the plasma half-life is 1.3 hours. The available plasma concentration time data at steady state in patients showed that anagrelide does not accumulate in plasma after repeated administration. The drug is extensively metabolized; less than 1% is recovered in the urine as anagrelide.
When a 0.5 mg dose of anagrelide was taken after food, its bioavailability (based on AUC values) was modestly reduced by an average of 13.8% and its plasma half-life slightly increased (to 1.8 hours), when compared with drug administered to the same subjects in the fasted state. The peak plasma level was lowered by an average of 45% and delayed by 2 hours.
A total of 942 patients with myeloproliferative disorders including 551 patients with Essential Thrombocythemia (ET), 117 patients with Polycythemia Vera (PV), 178 patients with Chronic Myelogenous Leukemia (CML), and 96 patients with other myeloproliferative disorders (OMPD), were treated with anagrelide in three clinical trials. Patients with OMPD included 87 patients who had Myeloid Metaplasia with Myelofibrosis (MMM), and 9 patients who had unknown myeloproliferative disorders.
Clinical Studies
Patients with ET, PV, CML, or MMM were diagnosed based on the following criteria:
|
Patients were enrolled in clinical trials if their platelet count was >/= 900,000/µL on two occasions or >/= 650,000/µL on two occasions with documentation of symptoms associated with thrombocythemia. The mean duration of anagrelide therapy for ET, PV, CML, and OMPD patients was 65, 67, 40, and 44 weeks, respectively; 23% of patients received treatment for 2 years. Patients were treated with anagrelide starting at doses of 0.5-2.0 mg every 6 hours. The dose was increased if the platelet count was still high, but to no more than 12 mg each day. Efficacy was defined as reduction of platelet count to or near physiologic levels (150,000-400,000/µL). The criteria for defining subjects as "responders" were reduction in platelets for at least 4 weeks to </=600,000/µL, or by at least 50% from baseline value. Subjects treated for less than 4 weeks were not considered evaluable. The results are depicted graphically below:
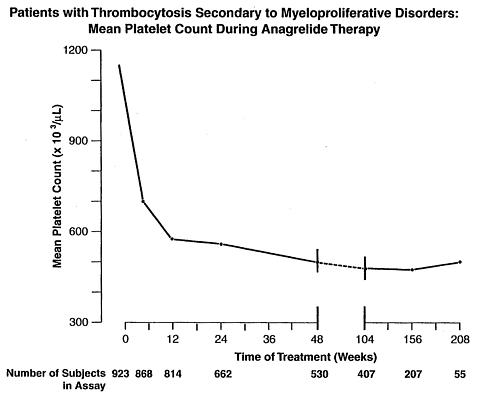 |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
AGRYLIN® was effective in phlebotomized patients as well as in patients treated with other concomitant therapies including hydroxyurea, aspirin, interferon, radioactive phosphorus, and alkylating agents.
AGRYLIN® Capsules are indicated for the treatment of patients with thrombocythemia, secondary to myeloproliferative disorders, to reduce the elevated platelet count and the risk of thrombosis and to ameliorate associated symptoms including thrombo-hemorrhagic events (see CLINICAL STUDIES , DOSAGE and ADMINISTRATION ).
Cardiovascular
Anagrelide should be used with caution in patients with known or suspected heart disease, and only if the potential benefits of therapy outweigh the potential risks. Because of the positive inotropic effects and side-effects of anagrelide, a pre-treatment cardiovascular examination is recommended along with careful monitoring during treatment. In humans, therapeutic doses of anagrelide may cause cardiovascular effects, including vasodilation, tachycardia, palpitations, and congestive heart failure.
Renal
It is recommended that patients with renal insufficiency (creatinine >/= 2mg/dL) receive anagrelide when, in the physician's judgment, the potential benefits of therapy outweigh the potential risks. These patients should be monitored closely for signs of renal toxicity while receiving anagrelide (see ADVERSE REACTIONS , Urogenital System ).
It is recommended that patients with evidence of hepatic dysfunction (bilirubin, SGOT, or measures of liver function >1.5 times the upper limit of normal) receive anagrelide when, in the physician's judgment, the potential benefits of therapy outweigh the potential risks. These patients should be monitored closely for signs of hepatic toxicity while receiving anagrelide (see ADVERSE REACTIONS , Hepatic System ).
Laboratory Tests: Anagrelide therapy requires close clinical supervision of the patient. While the platelet count is being lowered (usually during the first two weeks of treatment), blood counts (hemoglobin, white blood cells), liver function (SGOT, SGPT) and renal function (serum creatinine, BUN) should be monitored.
In 9 subjects receiving a single 5 mg dose of anagrelide, standing blood pressure fell an average of 22/15 mm Hg, usually accompanied by dizziness. Only minimal changes in blood pressure were observed following a dose of 2 mg.
Cessation of AGRYLIN® Treatment: In general, interruption of anagrelide treatment is followed by an increase in platelet count. After sudden stoppage of anagrelide therapy, the increase in platelet count can be observed within four days.
Drug Interactions: Bioavailability studies evaluating possible interactions between anagrelide and other drugs have not been conducted. The most common medications used concomitantly with anagrelide have been aspirin, acetaminophen, furosemide, iron, ranitidine, hydroxyurea, and allopurinol. The most frequently used concomitant cardiac medication has been digoxin. Although drug-to-drug interaction studies have not been conducted, there is no clinical evidence to suggest that anagrelide interacts with any of these compounds.
There is a single case report which suggests that sucralfate may interfere with anagrelide absorption.
Food has no clinically significant effect on the bioavailability of anagrelide.
Carcinogenesis, Mutagenesis, Impairment of Fertility: No long-term studies in animals have been performed to evaluate carcinogenic potential of anagrelide hydrochloride. Anagrelide hydrochloride was not genotoxic in the Ames test, the mouse lymphoma cell (L5178Y, TK +/- ) forward mutation test, the human lymphocyte chromosome aberration test, or the mouse micronucleus test. Anagrelide hydrochloride at oral doses up to 240 mg/kg/day (1,440 mg/m 2 /day, 195 times the recommended maximum human dose based on body surface area) was found to have no effect on fertility and reproductive performance of male rats. However, in female rats, at oral doses of 60 mg/kg/day (360 mg/m 2 /day, 49 times the recommended maximum human dose based on body surface area) or higher, it disrupted implantation when administered in early pregnancy and retarded or blocked parturition when administered in late pregnancy.
Pregnancy: Pregnancy Category C.
Nursing Mothers: It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reaction in nursing infants from anagrelide hydrochloride, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use: The safety and efficacy of anagrelide in patients under the age of 16 years have not been established. Myeloproliferative disorders are uncommon in pediatric patients. Anagrelide has been used successfully in 12 pediatric patients (age range 6.8 to 17.4 years; 6 male and 6 female), including 8 patients with ET, 2 patients with CML, 1 patient with PV, and 1 patient with OMPD. Patients were started on therapy with 0.5 mg qid to a maximum daily dose of 10 mg. The median duration of treatment was 18.1 months with a range of 3.1 to 92 months. Three patients received treatment for greater than three years.
Analysis of the adverse events in a population consisting of 942 patients diagnosed with myeloproliferative diseases of varying etiology (ET: 551; PV: 117; OMPD: 274) has shown that all disease groups have the same adverse event profile. While most reported adverse events during anagrelide therapy have been mild in intensity and have decreased in frequency with continued therapy, serious adverse events were reported in these patients. These include the following: congestive heart failure, myocardial infarction, cardiomyopathy, cardiomegaly, complete heart block, atrial fibrillation, cerebrovascular accident, pericarditis, pulmonary infiltrates, pulmonary fibrosis, pulmonary hypertension, pancreatitis, gastric/duodenal ulceration, and seizure.
Of the 942 patients treated with anagrelide for a mean duration of approximately 65 weeks, 161 (17%) were discontinued from the study because of adverse events or abnormal laboratory test results. The most common adverse events for treatment discontinuation were headache, diarrhea, edema, palpitation, and abdominal pain. Overall, the occurrence rate of all adverse events was 17.9 per 1,000 treatment days. The occurrence rate of adverse events increased at higher dosages of anagrelide.
The most frequently reported adverse reactions to anagrelide (in 5% or greater of 942 patients with myeloproliferative disease) in clinical trials were:
Headache .................................. 43.5%
Palpitations ................................ 26.1%
Diarrhea .................................... 25.7%
Asthenia .................................... 23.1%
Edema, other ............................. 20.6%
Nausea ...................................... 17.1%
Abdominal Pain .......................... 16.4%
Dizziness .................................... 15.4%
Pain, other ................................. 15.0%
Dyspnea .................................... 11.9%
Flatulence .................................. 10.2%
Vomiting ...................................... 9.7%
Fever ........................................... 8.9%
Peripheral Edema ..........................8.5%
Rash, including urticaria ................ 8.3%
Chest Pain ................................... 7.8%
Anorexia ...................................... 7.7%
Tachycardia ................................. 7.5%
Pharyngitis ................................... 6.8%
Malaise ........................................ 6.4%
Cough .......................................... 6.3%
Paresthesia ................................... 5.9%
Back Pain .................................... 5.9%
Pruritus ........................................... 5%
Dyspepsia .................................... 5.2%
Adverse events with an incidence of 1% to <5% included:
Body as a Whole System: Flu symptoms, chills, photosensitivity.
Cardiovascular System: Arrhythmia, hemorrhage, cardiovascular disease, angina pectoris, heart failure, postural hypotension, thrombosis, vasodilatation, migraine, syncope.
Digestive System: Constipation, GI distress, GI hemorrhage, gastritis, melena, aphthous stomatitis, eructation.
Hemic & Lymphatic System: Anemia, thrombocytopenia, ecchymosis, lymphadenopathy.
Platelet counts below 100,000/µL occurred in 84 patients (ET: 35; PV: 9; OMPD: 40), reduction below 50,000/µL occurred in 44 patients (ET: 7; PV: 6; OMPD: 31) while on anagrelide therapy. Thrombocytopenia promptly recovered upon discontinuation of anagrelide.
Hepatic System: Elevated liver enzymes were observed in 3 patients (ET: 2; OMPD: 1) during anagrelide therapy.
Musculoskeletal System: Arthralgia, myalgia, leg cramps.
Nervous System: Depression, somnolence, confusion, insomnia, hypertension, nervousness, amnesia.
Nutritional Disorders: Dehydration.
Respiratory System: Rhinitis, epistaxis, respiratory disease, sinusitis, pneumonia, bronchitis, asthma.
Skin and Appendages System: Skin disease, alopecia.
Special Senses: Amblyopia, abnormal vision, tinnitus, visual field abnormality, diplopia.
Urogenital System: Dysuria, Hematuria.
Renal abnormalities occurred in 15 patients (ET: 10; PV: 4; OMPD: 1). Six ET, 4 PV and 1 with OMPD experienced renal failure (approximately 1%) while on anagrelide treatment; in 4 cases, the renal failure was considered to be possibly related to anagrelide treatment. The remaining 11 were found to have pre-existing renal impairment. Doses ranged from 1.5-6.0 mg/day, with exposure periods of 2 to 12 months. No dose adjustment was required because of renal insufficiency.
The adverse event profile for patients in clinical trials on anagrelide therapy (in 5% or greater of 942 patients with myeloproliferative diseases) is shown in the following bar graph:
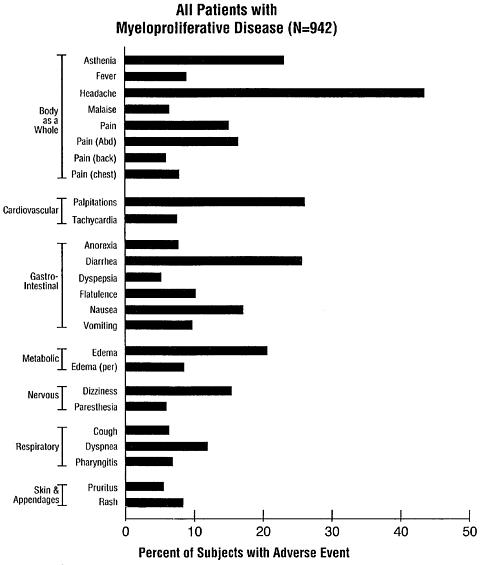 |
Single oral doses of anagrelide hydrochloride at 2,500, 1,500 and 200 mg/kg in mice, rats and monkeys, respectively, were not lethal. Symptoms of acute toxicity were: decreased motor activity in mice and rats and softened stools and decreased appetite in monkeys.
There are no reports of overdosage with anagrelide hydrochloride. Platelet reduction from anagrelide therapy is dose-related; therefore, thrombocytopenia, which can potentially cause bleeding, is expected from overdosage. Should overdosage occur, cardiac and central nervous system toxicity can also be expected.
Management and Treatment
In case of overdosage, close clinical supervision of the patient is required; this especially includes monitoring of the platelet count for thrombocytopenia. Dosage should be decreased or stopped, as appropriate, until the platelet count returns to within the normal range.
Treatment with AGRYLIN® Capsules should be initiated under close medical supervision. The recommended starting dosage of AGRYLIN® is 0.5 mg qid or 1 mg bid, which should be maintained for at least one week. Dosage should then be adjusted to the lowest effective dosage required to reduce and maintain platelet count below 600,000/µL, and ideally to the normal range. The dosage should be increased by not more than 0.5 mg/day in any one week. Dosage should not exceed 10 mg/day or 2.5 mg in a single dose (see PRECAUTIONS ). The decision to treat asymptomatic young adults with thrombocythemia secondary to myeloproliferative disorders should be individualized.
To monitor the effect of anagrelide and prevent the occurrence of thrombocytopenia, platelet counts should be performed every two days during the first week of treatment and at least weekly thereafter until the maintenance dosage is reached.
Typically, platelet count begins to respond within 7 to 14 days at the proper dosage. The time to complete response, defined as platelet count </= 600,000/µL, ranged from 4 to 12 weeks. Most patients will experience an adequate response at a dose of 1.5 to 3.0 mg/day. Patients with known or suspected heart disease, renal insufficiency, or hepatic dysfunction should be monitored closely.
AGRYLIN® is available as:
0.5 mg, opaque, white capsules imprinted "ROBERTS 063" in black ink:
NDC 54092-063-01 = bottle of 100
1 mg, opaque, gray capsules imprinted "ROBERTS 064" in black ink:
NDC 54092-064-01 = bottle of 100
Store from 15° to 25°C (59° to 77°F), in a light-resistant container.
ROBERTS® PHARMACEUTICALS
Manufactured for
Roberts Laboratories Inc., a subsidiary of
ROBERTS PHARMACEUTICAL CORP.
Eatontown, NJ 07724-2274, USA
by MALLINCKRODT INC.
Hobart, NY 13788
Copyright © 1999 Roberts Laboratories Inc.
Rev. 03/99 063 0117 007
 |