 |
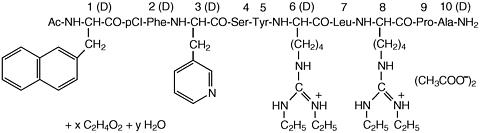
Antagon™ (ganirelix acetate) Injection is a synthetic decapeptide with high antagonistic activity against naturally occurring gonadotropin-releasing hormone (GnRH). Ganirelix acetate is derived from native GnRH with substitutions of amino acids at positions 1, 2, 3, 6, 8, and 10 to form the following molecular formula of the peptide: N-acetyl-3-(2-napthyl)-D-alanyl-4-chloro-D-phenylalanyl-3-(3-pyridyl)-D-alanyl-L-seryl-L-tyrosyl-N 9 ,N 10 -diethyl- D-homoarginyl-L-leucyl-N 9 ,N 10 -diethyl-L-homoarginyl-L-prolyl-D-alanylamide acetate. The molecular weight for ganirelix acetate is 1570.4 as an anhydrous free base. The structural formula is as follows:
Ganirelix acetate
 |
Antagon™ is supplied as a colorless, sterile, ready-to-use, aqueous solution intended for SUBCUTANEOUS administration only. Each sterile, prefilled syringe contains 250 µg/0.5 mL of ganirelix acetate, 0.1 mg glacial acetic acid, 23.5 mg mannitol, and water for injection adjusted to pH 5.0 with acetic acid, NF and/or sodium hydroxide, NF.
The pulsatile release of GnRH stimulates the synthesis and secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). The frequency of LH pulses in the mid and late follicular phase is approximately 1 pulse per hour. These pulses can be detected as transient rises in serum LH. At midcycle, a large increase in GnRH release results in an LH surge. The midcycle LH surge initiates several physiologic actions including: ovulation, resumption of meiosis in the oocyte, and luteinization. Luteinization results in a rise in serum progesterone with an accompanying decrease in estradiol levels.
Antagon™ (ganirelix acetate) Injection acts by competitively blocking the GnRH receptors on the pituitary gonadotroph and subsequent transduction pathway. It induces a rapid, reversible suppression of gonadotropin secretion. The suppression of pituitary LH secretion by Antagon™ is more pronounced than that of FSH. An initial release of endogenous gonadotropins has not been detected with Antagon™, which is consistent with an antagonist effect. Upon discontinuation of Antagon™, pituitary LH and FSH levels are fully recovered within 48 hours.
The pharmacokinetic parameters of single and multiple injections of Antagon™ (ganirelix acetate) Injection in healthy adult females are summarized in Table I. Steady state serum concentrations are reached after 3 days of treatment. The pharmacokinetics of ganirelix acetate are dose-proportional in the dose range of 125 to 500 µg.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Absorption
Ganirelix acetate is rapidly absorbed following subcutaneous injection with maximum serum concentrations reached approximately one hour after dosing. The mean absolute bioavailability of Antagon™ following a single 250 µg subcutaneous injection to healthy female volunteers is 91.1%.
The mean (SD) volume of distribution of Antagon™ in healthy females following intravenous administration of a single 250 µg dose is 43.7 (11.4) liters (L). In vitro protein binding to human plasma is 81.9%.
Following single dose intravenous administration of radiolabeled Antagon™ to healthy female volunteers, Antagon™ is the major compound present in the plasma (50-70% of total radioactivity in the plasma) up to 4 hours and urine (17.1-18.4% of administered dose) up to 24 hours. Antagon™ is not found in the feces. The 1-4 peptide and 1-6 peptide of Antagon™ are the primary metabolites observed in the feces.
Excretion
On average, 97.2% of the total radiolabeled Antagon™ dose is recovered in the feces and urine (75.1% and 22.1%, respectively) over 288 h following intravenous single dose administration of 1 mg [ 14 C]-ganirelix acetate. Urinary excretion is virtually complete in 24 h, whereas fecal excretion starts to plateau 192 h after dosing.
The pharmacokinetics of ganirelix acetate have not been determined in special populations such as geriatric, pediatric, renally impaired and hepatically impaired patients (see PRECAUTIONS ).
Formal in vivo or in vitro drug-drug interaction studies have not been conducted (see PRECAUTIONS ). Since Antagon™ can suppress the secretion of pituitary gonadotropins, dose adjustments of exogenous gonadotropins may be necessary when used during controlled ovarian hyperstimulation (COH).
The efficacy of Antagon™ (ganirelix acetate) Injection was established in two adequate and well-controlled clinical studies which included women with normal endocrine and pelvic ultra-sound parameters. The studies intended to exclude subjects with polycystic ovary syndrome (PCOS) and subjects with low or no ovarian reserve. One cycle of study medication was administered to each randomized subject. For both studies, the administration of exogenous recombinant FSH [Follistim® (follitropin beta for injection)] 150 IU daily was initiated on the morning of Day 2 or 3 of a natural menstrual cycle. Antagon™ was administered on the morning of Day 7 or 8 (Day 6 of recombinant FSH administration). The dose of recombinant FSH administered was adjusted according to individual responses starting on the day of initiation of Antagon™. Both recombinant FSH and Antagon™ were continued daily until at least three follicles were 17 mm or greater in diameter at which time hCG [Pregnyl® (chorionic gonadotropin for injection, USP)] was administered. Following hCG administration, Antagon™ and recombinant FSH administration were discontinued. Oocyte retrieval, followed by in vitro fertilization (IVF) or intracytoplasmatic sperm injection (ICSI), was subsequently performed.
In a multicenter, double-blind, randomized, dose-finding study, the safety and efficacy of Antagon™ were evaluated for the prevention of LH surges in women undergoing COH with recombinant FSH. Antagon™ doses ranging from 62.5 µg to 2000 µg and recombinant FSH were administered to 332 patients undergoing COH for IVF (see TABLE II). Median serum LH on the day of hCG administration decreased with increasing doses of Antagon™. Median serum E 2 (17(beta)-estradiol) on the day of hCG administration was 1475, 1110, and 1160 pg/mL for the 62.5, 125, and 250 µg doses, respectively. Lower peak serum E 2 levels of 823, 703, and 441 pg/mL were seen at higher doses of Antagon™ 500, 1000, and 2000 µg, respectively. The highest pregnancy and implantation rates were achieved with the 250 µg dose of Antagon™ as summarized in Table II.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Transient LH rises alone were not deleterious to achieving pregnancy with Antagon™ at doses of 125 µg (3/6 subjects) and 250 µg (1/1 subjects). In addition, none of the subjects with LH rises >/= 10 mIU/mL had premature luteinization indicated by a serum progesterone above 2 ng/mL.
A multicenter, open-label, randomized study was conducted to assess the efficacy and safety of Antagon™ in women undergoing COH. Follicular phase treatment with Antagon™ 250 µg was studied using a luteal phase GnRH agonist as a reference treatment. A total of 463 subjects were treated with Antagon™ by subcutaneous injection once daily starting on Day 6 of recombinant FSH treatment. Recombinant FSH was maintained at 150 IU for the first 5 days of ovarian stimulation and was then adjusted by the investigator on the sixth day of gonadotropin use according to individual responses. The results for the Antagon™ arm are summarized in Table III.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The mean number of days of Antagon™ treatment was 5.4 (2-14). There was no incidence of drug related allergic reactions within the adequate and well-controlled clinical studies.
LH Surges
The midcycle LH surge initiates several physiologic actions including: ovulation, resumption of meiosis in the oocyte, and luteinization. In 463 subjects administered Antagon™ 250 µg, a premature LH surge prior to hCG administration, (LH rise >/= 10 mIU/mL with a significant rise in serum progesterone > 2 ng/mL, or a significant decline in serum estradiol) occurred in less than 1% of subjects.
Antagon™ (ganirelix acetate) Injection is indicated for the inhibition of premature LH surges in women undergoing controlled ovarian hyperstimulation.
Antagon™ (ganirelix acetate) Injection is contraindicated under the following conditions:
Antagon™ (ganirelix acetate) Injection should be prescribed by physicians who are experienced in infertility treatment. Before starting treatment with Antagon™, pregnancy must be excluded. Safe use of Antagon™ during pregnancy has not been established (see CONTRAINDICATIONS and PRECAUTIONS ).
Caution is advised in patients with hypersensitivity to GnRH. These patients should be carefully monitored after the first injection. Anaphylactic reactions or ganirelix antibody formation have not been reported in the clinical trials for Antagon™ (ganirelix acetate) Injection.
The packaging of this product contains natural rubber latex which may cause allergic reactions.
Prior to therapy with Antagon™ (ganirelix acetate) Injection, patients should be informed of the duration of treatment and monitoring procedures that will be required. The risk of possible adverse reactions should be discussed (see ADVERSE REACTIONS ).
Antagon™ should not be prescribed if the patient is pregnant.
A neutrophil count >/= 8.3 ( × 10 9 /L) was noted in 11.9% (up to 16.8 × 10 9 /L) of all subjects treated within the adequate and well-controlled clinical trials. In addition, downward shifts within the Antagon™ (ganirelix acetate) Injection group were observed for hematocrit and total bilirubin. The clinical significance of these findings was not determined.
No formal drug-drug interaction studies have been performed.
Long-term toxicity studies in animals have not been performed with Antagon™ (ganirelix acetate) Injection to evaluate the carcinogenic potential of the drug. Antagon™ did not induce a mutagenic response in the Ames test (S. typhimurium and E. coli) or produce chromosomal aberrations in in vitro assay using Chinese Hamster Ovary cells.
Pregnancy Category X
Antagon™ (ganirelix acetate) Injection is contraindicated in pregnant women. When administered from Day 7 to near term to pregnant rats and rabbits at doses up to 10 and 30 µg/day (approximately 0.4 to 3.2 times the human dose based on body surface area), Antagon™ increased the incidence of litter resorption. There was no increase in fetal abnormalities. No treatment related changes in fertility, physical, or behavioral characteristics were observed in the offspring of female rats treated with Antagon™ during pregnancy and lactation.
The effects on fetal resorption are logical consequences of the alteration in hormonal levels brought about by the antigonadotrophic properties of this drug and could result in fetal loss in humans. Therefore, this drug should not be used in pregnant women (see CONTRAINDICATIONS ).
Antagon™ (ganirelix acetate) Injection should not be used by lactating women. It is not known whether this drug is excreted in human milk.
Clinical studies with Antagon™ (ganirelix acetate) Injection did not include a sufficient number of subjects aged 65 and over.
The safety of Antagon™ (ganirelix acetate) Injection was evaluated in two randomized, parallel-group, multicenter controlled clinical studies. Treatment duration for Antagon™ ranged from 1 to 14 days. Table IV represents adverse events (AEs) from first day of Antagon™ administration until confirmation of pregnancy by ultrasound at an incidence of >/= 1% of Antagon™-treated subjects without regard to causality.
|
Congenital Anomalies
Ongoing clinical follow-up studies of 283 newborns of women administered Antagon™ were reviewed. There were three neonates with major congenital anomalies and 18 neonates with minor congenital anomalies. The major congenital anomalies were: hydrocephalus/meningocele, omphalocele, and Beckwith-Wiedemann Syndrome. The minor congenital anomalies were: nevus, skin tags, sacral sinus, hemangioma, torticollis/asymmetric skull, talipes, supernumerary digit finger, hip subluxation, torticollis/high palate, occiput/abnormal hand crease, hernia umbilicalis, hernia inguinalis, hydrocele, undescended testis, and hydronephrosis. The causal relationship between these congenital anomalies and Antagon™ is unknown. Multiple factors, genetic and others (including, but not limited to ICSI, IVF, gonadotropins, progesterone) may confound ART (Assisted Reproductive Technology) procedures.
There have been no reports of overdosage with Antagon™ (ganirelix acetate) Injection in humans.
After initiating FSH therapy on Day 2 or 3 of the cycle, Antagon™ (ganirelix acetate) Injection 250 µg may be administered subcutaneously once daily during the early to mid follicular phase. By taking advantage of endogenous pituitary FSH secretion, the requirement for exogenously administered FSH may be reduced. Treatment with Antagon™ should be continued daily until the day of hCG administration. When a sufficient number of follicles of adequate size are present, as assessed by ultrasound, final maturation of follicles is induced by administering hCG. The administration of hCG should be withheld in cases where the ovaries are abnormally enlarged on the last day of FSH therapy to reduce the chance of developing OHSS.
Antagon™ (ganirelix acetate) Injection is supplied in:
Disposable, sterile, prefilled 1 mL glass syringes containing 250 µg/0.5 mL of ganirelix acetate. Each Antagon™ sterile, prefilled syringe is affixed with a 27 gauge × ½ inch needle and is blister-packed.
Single syringe NDC 0052-0301-51
Box of 5 NDC 0052-0301-61
Box of 50 NDC 0052-0301-71
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F) [see USP Controlled Room Temperature]. Protect from light.
Rx only
Manufactured for Organon Inc.
by Vetter Pharma-Fertigung GmbH & Co. KG
Ravensburg, Germany
and packaged by Organon (Ireland) Ltd, Swords Co.
Dublin, Ireland
5310194 6/99 08