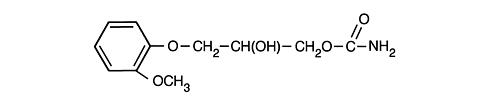 |
|
||||||
Inactive Ingredients: Corn Starch, FD&C Red 3, Magnesium Stearate, Povidone, Sodium Lauryl Sulfate, Sodium Starch Glycolate, Stearic Acid.
Methocarbamol has the following structural formula and chemical name:
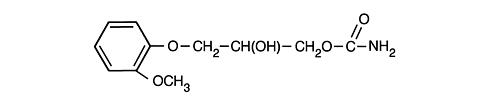 |
3-(2-Methoxyphenoxy)-1,2-propanediol
1-Carbamate
Robaxisal provides a double approach to the management of discomforts associated with musculoskeletal disorders.
The mechanism of action of methocarbamol in humans has not been established, but may be due to general central nervous system depression. It has no direct action on the contractile mechanism of striated muscle, the motor end plate or the nerve fiber.
ASPIRIN
Aspirin is a mild analgesic with anti-inflammatory and antipyretic activity.
Robaxisal is indicated as an adjunct to rest, physical therapy, and other measures for the relief of discomfort associated with acute, painful musculoskeletal conditions. The mode of action of methocarbamol has not been clearly identified but may be related to its sedative properties. Methocarbamol does not directly relax tense skeletal muscles in man.
Hypersensitivity to methocarbamol or aspirin.
Since methocarbamol may possess a general central nervous system depressant effect, patients receiving Robaxisal should be cautioned about combined effects with alcohol and other CNS depressants.
Products containing aspirin should be administered with caution to patients with gastritis or peptic ulceration, or those receiving hypoprothrombinemic anticoagulants.
Methocarbamol may cause a color interference in certain screening tests for 5-hydroxyindoleacetic acid (5-HIAA) and vanillylmandelic acid (VMA).
Safe use of Robaxisal has not been established with regard to possible adverse effects upon fetal development. Therefore, Robaxisal should not be used in women who are or may become pregnant and particularly during early pregnancy unless in the judgment of the physician the potential benefits outweigh the possible hazards.
NURSING MOTHERS
It is not known whether methocarbamol is secreted in human milk; however, aspirin does appear in human milk in moderate amounts. It can produce a bleeding tendency either by interfering with the function of the infant' platelets or by decreasing the amount of prothrombin in the blood. The risk is minimal if the mother takes the aspirin just after nursing and if the infant has an adequate store of vitamin K. As a general rule, nursing should not be undertaken while a patient is on a drug.
PEDIATRIC USE
Safety and effectiveness in children 12 years of age and below have not been established.
USE IN ACTIVITIES REQUIRING MENTAL ALERTNESS
Robaxisal may rarely cause drowsiness. Until the patient' response has been determined, he should be cautioned against the operation of motor vehicles or dangerous machinery.
The most frequent adverse reaction to methocarbamol is dizziness or lightheadedness and nausea. This occurs in about one in 20-25 patients. Less frequent reactions are drowsiness, blurred vision, headache, fever, allergic manifestations such as urticaria, pruritus, and rash.
Adverse reactions that have been associated with the use of aspirin include: nausea and other gastrointestinal discomfort, gastritis, gastric erosion, vomiting, constipation, diarrhea, angio-edema, asthma, rash, pruritus, urticaria.
Gastrointestinal discomfort may be minimized by taking Robaxisal with food.
Adults and children over 12 years of age: Two tablets four times daily. Three tablets four times daily may be used in severe conditions for one to three days in patients who are able to tolerate salicylates. These dosage recommendations provide respectively 3.2 and 4.8 grams of methocarbamol per day.
Toxicity due to overdosage of methocarbamol is unlikely; however, acute overdosage of aspirin may cause symptoms of salicylate intoxication.
TREATMENT OF OVERDOSAGE
Supportive therapy for 24 hours, as methocarbamol is excreted within that time. If salicylate intoxication occurs, especially in children, the hyperpnea may be controlled with sodium bicarbonate. Judicious use of 5% CO 2 with 95% O 2 may be of benefit. Abnormal electrolyte patterns should be corrected with appropriate fluid therapy.
Robaxisal® is supplied as pink and white laminated, compressed tablets in bottles of 100 (NDC 0031-7469-63) and 500 (NDC 0031-7469-70).
Store at controlled room temperature, between 20° and 25°C (68° and 77°F).
Dispense in well-closed container.
Manufactured by:
Pharmaceutical Division
A.H. Robins Company
Richmond, VA 23220
CI 4697-1 Issued February 6, 1997
 |