 |
Bicillin C-R 900/300 (penicillin G benzathine and penicillin G procaine suspension) contains the equivalent of 900,000 units of penicillin G as the benzathine and 300,000 units of penicillin G as the procaine salts. It is available for deep intramuscular injection.
Penicillin G benzathine is prepared by the reaction of dibenzylethylene diamine with two molecules of penicillin G. It is chemically designated as (2 S , 5 R , 6 R )-3,3-Dimethyl-7-oxo-6-(2-phenylacetamido)-4-thia-1-azabicyclo[3.2.0] heptane-2-carboxylic acid compound with N,N' -dibenzylethylenediamine (2:1), tetrahydrate. It occurs as a white, crystalline powder and is very slightly soluble in water and sparingly soluble in alcohol.
Penicillin G procaine, (2 S , 5 R , 6 R )-3,3-Dimethyl-7-oxo-6-(2-phenylacetamido)-4-thia-1-azabicyclo[3.2.0] heptane-2- carboxylic acid compound with 2-(diethylamino)ethyl p -aminobenzoate compound (1:1) monohydrate, is an equimolar salt of procaine and penicillin G. It occurs as white crystals or a white, microcrystalline powder and is slightly soluble in water.
Each TUBEX ® cartridge (2 mL size) contains the equivalent to 1,200,000 units of penicillin G as follows: penicillin G benzathine equivalent to 900,000 units of penicillin G and penicillin G procaine equivalent to 300,000 units of penicillin G in a stabilized aqueous suspension with sodium citrate buffer; and as w/v, approximately 0.5% lecithin, 0.55% carboxymethylcellulose, 0.55% povidone, 0.1% methylparaben, and 0.01% propylparaben.
Bicillin C-R 900/300 suspension in TUBEX formulation is viscous and opaque. Read CONTRAINDICATIONS , , PRECAUTIONS , and DOSAGE AND ADMINISTRATION sections prior to use.
General
Penicillin G benzathine and penicillin G procaine have a low solubility and, thus, the drugs are slowly released from intramuscular injection sites. The drugs are hydrolyzed to penicillin G. This combination of hydrolysis and slow absorption results in blood serum levels much lower but more prolonged than other parenteral penicillins. Intramuscular administration of 1,200,000 units of Bicillin C-R 900/300 in patients weighing 100 to 140 lbs. usually produces average blood levels of 0.24 units/mL at 24 hours, 0.039 units/mL at 7 days, and 0.024 units/mL at 10 days.
Approximately 60% of penicillin G is bound to serum protein. The drug is distributed throughout the body tissues in widely varying amounts. Highest levels are found in the kidneys with lesser amounts in the liver, skin, and intestines. Penicillin G penetrates into all other tissues and the spinal fluid to a lesser degree. With normal kidney function, the drug is excreted rapidly by tubular excretion. In neonates and young infants and in individuals with impaired kidney function, excretion is considerably delayed.
Penicillin G exerts a bactericidal action against penicillin-susceptible microorganisms during the stage of active multiplication. It acts through the inhibition of biosynthesis of cell-wall mucopeptide. It is not active against the penicillinase-producing bacteria, which include many strains of staphylococci.
The following in vitro data are available, but their clinical significance is unknown. Penicillin G exerts high in vitro activity against staphylococci (except penicillinase-producing strains), streptococci (Groups A, C, G, H, L, and M), and pneumococci. Other organisms susceptible to penicillin G are Neisseria gonorrhoeae, Corynebacterium diphtheriae, Bacillus anthracis, Clostridia species, Actinomyces bovis, Streptobacillus moniliformis, Listeria monocytogenes, and Leptospira species. Treponema pallidum is extremely susceptible to the bactericidal action of penicillin G.
Susceptibility Test: If the Kirby-Bauer method of disc susceptibility is used, a 10-unit penicillin disc should give a zone greater than 28 mm when tested against a penicillin-susceptible bacterial strain.
Bicillin C-R 900/300 is indicated in the treatment of infections as described below that are susceptible to serum levels characteristic of this particular dosage form. Therapy should be guided by bacteriological studies (including susceptibility testing) and by clinical response.
Bicillin C-R 900/300 is indicated in the treatment of the following in pediatric patients:
Moderately severe to severe infections of the upper-respiratory tract, scarlet fever, erysipelas, and skin and soft-tissue infections due to susceptible streptococci.
NOTE: Streptococci in Groups A, C, G, H, L, and M are very susceptible to penicillin G. Other groups, including Group D (enterococci), are resistant. Penicillin G sodium or potassium is recommended for streptococcal infections with bacteremia.
Moderately severe pneumonia and otitis media due to susceptible pneumococci.
NOTE: Severe pneumonia, empyema, bacteremia, pericarditis, meningitis, peritonitis, and arthritis of pneumococcal etiology are better treated with penicillin G sodium or potassium during the acute stage.
When high, sustained serum levels are required, penicillin G sodium or potassium, either IM or IV, should be used. This drug should not be used in the treatment of venereal diseases, including syphilis, gonorrhea, yaws, bejel, and pinta.
A previous hypersensitivity reaction to any penicillin or to procaine is a contraindication.
Do not inject into or near an artery or nerve.
The combination of penicillin G benzathine and penicillin G procaine should only be prescribed for the indications listed in this insert.
SERIOUS AND OCCASIONALLY FATAL HYPERSENSITIVITY (ANAPHYLACTIC) REACTIONS HAVE BEEN REPORTED IN PATIENTS ON PENICILLIN THERAPY. THESE REACTIONS ARE MORE LIKELY TO OCCUR IN INDIVIDUALS WITH A HISTORY OF PENICILLIN HYPERSENSITIVITY AND/OR A HISTORY OF SENSITIVITY TO MULTIPLE ALLERGENS. THERE HAVE BEEN REPORTS OF INDIVIDUALS WITH A HISTORY OF PENICILLIN HYPERSENSITIVITY WHO HAVE EXPERIENCED SEVERE REACTIONS WHEN TREATED WITH CEPHALOSPORINS. BEFORE INITIATING THERAPY WITH BICILLIN C-R 900/300, CAREFUL INQUIRY SHOULD BE MADE CONCERNING PREVIOUS HYPERSENSITIVITY REACTIONS TO PENICILLINS, CEPHALOSPORINS AND OTHER ALLERGENS, IF AN ALLERGIC REACTION OCCURS, BICILLIN C-R 900/300 SHOULD BE DISCONTINUED AND APPROPRIATE THERAPY INSTITUTED. SERIOUS ANAPHYLACTIC REACTIONS REQUIRE IMMEDIATE EMERGENCY TREATMENT WITH EPINEPHRINE. OXYGEN, INTRAVENOUS STEROIDS AND AIRWAY MANAGEMENT, INCLUDING INTUBATION, SHOULD ALSO BE ADMINISTERED AS INDICATED.
Pseudomembranous colitis has been reported with nearly all antibacterial agents, including penicillin, and may range in severity from mild to life-threatening. Therefore, it is important to consider this diagnosis in patients who present with diarrhea subsequent to the administration of any antibacterial agent.
Treatment with antibacterial agents alters the normal flora of the colon and may permit overgrowth of clostridia. Studies indicate that a toxin produced by Clostridium difficile is one primary cause of "antibiotic colitis".
After the diagnosis of pseudomembranous colitis has been established, appropriate therapeutic measures should be initiated. Mild cases of pseudomembranous colitis usually respond to drug discontinuation alone. In moderate to severe cases, consideration should be given to management with fluids and electrolytes, protein supplementation, and treatment with an antibacterial drug clinically effective against C. difficile colitis
Inadvertent intravascular administration, including inadvertent direct intra-arterial injection or injection immediately adjacent to arteries, of Bicillin C-R 900/300 and other penicillin preparations has resulted in severe neurovascular damage, including transverse myelitis with permanent paralysis, gangrene requiring amputation of digits and more proximal portions of extremities, and necrosis and sloughing at and surrounding the injection site. Such severe effects have been reported following injections into the buttock, thigh, and deltoid areas. Other serious complications of suspected intravascular administration which have been reported include immediate pallor, mottling, or cyanosis of the extremity both distal and proximal to the injection site, followed by bleb formation; severe edema requiring anterior and/or posterior compartment fasciotomy in the lower extremity. The above-described severe effects and complications have most often occurred in infants and small children. Prompt consultation with an appropriate specialist is indicated if any evidence of compromise of the blood supply occurs at, proximal to, or distal to the site of injection. 1 - 9 See CONTRAINDICATIONS , PRECAUTIONS , and DOSAGE AND ADMINISTRATION sections.
Quadriceps femoris fibrosis and atrophy have been reported following repeated intramuscular injections of penicillin preparations into the anterolateral thigh.
Injection into or near a nerve may result in permanent neurological damage.
General
Penicillin should be used with caution in individuals with histories of significant allergies and/or asthma.
Care should be taken to avoid intravenous or intra-arterial administration, or injection into or near major peripheral nerves or blood vessels, since such injections may produce neurovascular damage. See CONTRAINDICATIONS , , and DOSAGE AND ADMINISTRATION sections.
A small percentage of patients are sensitive to procaine. If there is a history of sensitivity, make the usual test: Inject intradermally 0.1 mL of a 1 to 2 percent procaine solution. Development of an erythema, wheal, flare, or eruption indicates procaine sensitivity. Sensitivity should be treated by the usual methods, including barbiturates, and procaine penicillin preparations should not be used. Antihistaminics appear beneficial in treatment of procaine reactions.
The use of antibiotics may result in overgrowth of nonsusceptible organisms. Constant observation of the patient is essential. If new infections due to bacteria or fungi appear during therapy, the drug should be discontinued and appropriate measures taken.
Whenever allergic reactions occur, penicillin should be withdrawn unless, in the opinion of the physician, the condition being treated is life-threatening and amenable only to penicillin therapy.
In prolonged therapy with penicillin, and particularly with high-dosage schedules, periodic evaluation of the renal and hematopoietic systems is recommended.
Laboratory Tests
In streptococcal infections, therapy must be sufficient to eliminate the organism; otherwise, the sequelae of streptococcal disease may occur. Cultures should be taken following completion of treatment to determine whether streptococci have been eradicated.
Tetracycline, a bacteriostatic antibiotic, may antagonize the bactericidal effect of penicillin, and concurrent use of these drugs should be avoided.
Concurrent administration of penicillin and probenecid increases and prolongs serum penicillin levels by decreasing the apparent volume of distribution and slowing the rate of excretion by competitively inhibiting renal tubular secretion of penicillin.
Pregnancy Category B
Reproduction studies performed in the mouse, rat, and rabbit have revealed no evidence of impaired fertility or harm to the fetus due to penicillin G. Human experience with the penicillins during pregnancy has not shown any positive evidence of adverse effects on the fetus. There are, however, no adequate and well-controlled studies in pregnant women showing conclusively that harmful effects of these drugs on the fetus can be excluded. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
Soluble penicillin G is excreted in breast milk. Caution should be exercised when penicillin G benzathine and penicillin G procaine are administered to a nursing woman.
Carcinogeneses, Mutagenesis, Impairment of Fertility
No long-term animal studies have been conducted with these drugs.
Pediatric Use
See and DOSAGE AND ADMINISTRATION .
As with other penicillins, untoward reactions of the sensitivity phenomena are likely to occur, particularly in individuals who have previously demonstrated hypersensitivity to penicillins or in those with a history of allergy, asthma, hay fever, or urticaria.
The following have been reported with parenteral penicillin G:
General: Hypersensitivity reactions including the following: skin eruptions (maculopapular to exfoliative dermatitis), urticaria, laryngeal edema, fever, eosinophilia; other serum-sickness-like reactions (including chills, fever, edema, arthralgia, and prostration); anaphylaxis including shock and death. NOTE: Urticaria, other skin rashes, and serum sickness-like reactions may be controlled with antihistamines and, if necessary, systemic corticosteroids.
Whenever such reactions occur, penicillin G should be discontinued unless, in the opinion of the physician, the condition being treated is life-threatening and amenable only to therapy with penicillin G. Serious anaphylactic reactions require immediate emergency treatment with epinephrine. Oxygen, intravenous steroids, and airway management, including intubation, should also be administered as indicated.
Gastrointestinal Pseudomembranous colitis. Onset of pseudomembranous colitis symptoms may occur during or after antibacterial treatment. (See .)
Hematologic: Hemolytic anemia, leukopenia, thrombocytopenia.
Neurologic: Neuropathy
Urogenital: Nephropathy
The following adverse events have been temporally associated with parenteral administration of penicillin G benzathine:
Body as a Whole: Hypersensitivity reactions including allergic vasculitis, pruritus, fatigue, asthenia, and pain; aggravation of existing disorder; headache.
Cardiovascular: Cardiac arrest; hypotension; tachycardia; palpitations; pulmonary hypertension; pulmonary embolism; vasodilatation; vasovagal reaction; cerebrovascular accident; syncope.
Gastrointestinal: Nausea, vomiting; blood in stool; intestinal necrosis.
Hemic and Lymphatic: Lymphadenopathy.
Injection Site: Injection site reactions including pain, inflammation, lump, abscess, necrosis, edema, hemorrhage, cellulitis, hypersensitivity, atrophy, ecchymosis, and skin ulcer. Neurovascular reactions including warmth, vasospasm, pallor, mottling, gangrene, numbness of the extremities, cyanosis of the extremities, and neurovascular damage.
Metabolic Elevated BUN, creatinine, and SGOT.
Musculoskeletal: Joint disorder; periostitis; exacerbation of arthritis; myoglobinuria; rhabdomyolysis.
Nervous System: Nervousness; tremors; dizziness; somnolence; confusion; anxiety; euphoria; transverse myelitis; seizures; coma. A syndrome manifested by a variety of CNS symptoms such as severe agitation with confusion, visual and auditory hallucinations, and a fear of impending death (Hoigne's syndrome), has been reported after administration of penicillin G procaine and, less commonly, after injection of the combination of penicillin G benzathine and penicillin G procaine. Other symptoms associated with this syndrome, such as psychosis, seizures, dizziness, tinnitus, cyanosis, palpitations, tachycardia, and/or abnormal perception in taste, also may occur.
Respiratory Hypoxia; apnea; dyspnea.
Skin: Diaphoresis.
Special Senses: Blurred vision; blindness.
Urogenital: Neurogenic bladder; hematuria; proteinuria; renal failure; impotence; priapism.
Penicillin in overdosage has the potential to cause neuromuscular hyperirritability or convulsive seizures.
Administer by DEEP, INTRAMUSCULAR INJECTION in the upper, outer quadrant of the buttock. In neonates, infants and small children, the midlateral aspect of the thigh may be preferable. When doses are repeated, vary the injection site.
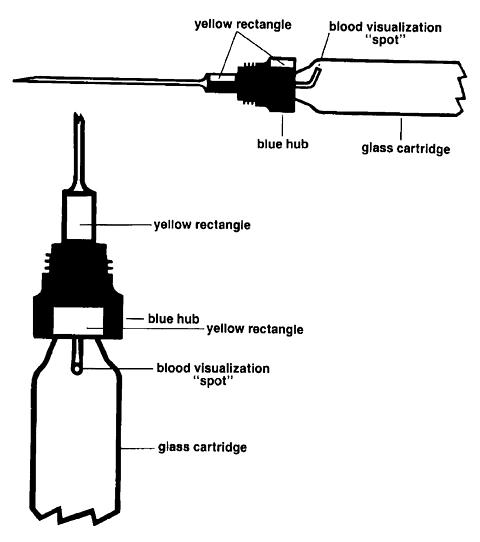
The Wyeth-Ayerst TUBEX cartridge for this product incorporates several features that are designed to facilitate the visualization of blood on aspiration if a blood vessel is inadvertently entered.
 |
The design of this cartridge is such that blood which enters its needle will be quickly visualized as a red or dark-colored "spot." This "spot" will appear on the barrel of the glass cartridge immediately proximal to the blue hub. The TUBEX is designed with two orientation marks, in order to determine where the "spot" can be seen. First insert and secure the cartridge in the TUBEX injector in the usual fashion. Locate the yellow rectangle at the base of the blue hub. This yellow rectangle is aligned with the blood visualization "spot." An imaginary straight line, drawn from this yellow rectangle to the shoulder of the glass cartridge, will point to the area on the cartridge where the "spot" can be visualized. When the needle cover is removed, a second yellow rectangle will be visible. The second yellow rectangle is also aligned with the blood visualization "spot" to assist the operator in locating the "spot." If the 2 mL metal or plastic syringe is used, the glass cartridge should be rotated by turning the plunger of the syringe clockwise until the yellow rectangle is visualized. If the 1 mL metal syringe is used, it will not be possible to continue to rotate the glass cartridge clockwise once it is properly engaged and fully threaded; it can, however, then be rotated counterclockwise as far as necessary to properly orient the yellow rectangles and locate the observation area. (In this same area in some cartridges, a dark spot may sometimes be visualized prior to injection. This is the proximal end of the needle and does not represent a foreign body in, or other abnormality of, the suspension.)
Thus, before the needle is inserted into the selected muscle, it is important for the operator to orient the yellow rectangle so that any blood which may enter after needle insertion and during aspiration can be visualized in the area on the cartridge where it will appear and not be obscured by any obstructions.
After selection of the proper site and insertion of the needle into the selected muscle, aspirate by pulling back on the plunger. While maintaining negative pressure for 2 to 3 seconds, carefully observe the neck of the glass TUBEX cartridge immediately proximal to the blue plastic needle hub for appearance of blood or any discoloration.
Blood or "typical blood color" may not be seen if a blood vessel has been entered--only a mixture of blood and Bicillin C-R 900/300. The appearance of any discoloration is reason to withdraw the needle and discard the TUBEX . If it is elected to inject at another site, a new TUBEX cartridge should be used. If no blood or discoloration appears, inject the contents of the TUBEX slowly. Discontinue delivery of the dose if the subject complains of severe immediate pain at the injection site or if, especially in neonates, infants and young children, symptoms or signs occur suggesting onset of severe pain.
Some TUBEX cartridges may contain a small air bubble which may be disregarded, since it does not affect administration of the product. DO NOT clear any air bubbles from the cartridge or needle as this may interfere with the visualization of any blood or discoloration during aspiration.
Because of the high concentration of suspended material in this product, the needle may be blocked if the injection is not made at a slow, steady rate.
Streptococcal Infections
Group A Infections of the upper-respiratory tract, skin and soft-tissue infections, scarlet fever, and erysipelas: single injection of Bicillin C-R 900/300 is usually sufficient for the treatment of Group A streptococcal infections in pediatric patients.
Pneumococcal Infections (except pneumococcal meningitis)
One TUBEX Bicillin C-R 900/300 repeated at 2- or 3-day intervals until the temperature is normal for 48 hours. Other forms of penicillin may be necessary for severe cases.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Bicillin® C-R 900/300 (penicillin G benzathine and penicillin G procaine suspension) is supplied in 2 mL size TUBEX® Sterile Cartridge-Needle Units in packages of 10 TUBEX® as follows:
1,200,000 units per TUBEX® (21 gauge, thin-wall 1 inch needle for pediatric use), NDC 0008-0079-36.
1,200,000 units per TUBEX® (21 gauge, thin-wall 1-1/4 inch needle), NDC 0008-0079-35.
Keep from freezing.
Manufactured by:
Wyeth Laboratories
A Wyeth-Ayerst Company
Philadelphia, PA 19101
Distributed by:
Monarch Pharmaceuticals, Inc.
Bristol, TN 37620