 |
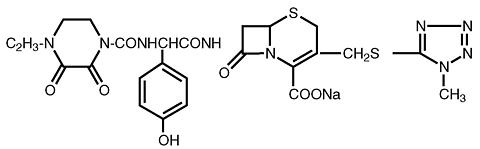
CEFOBID® (sterile cefoperazone), formerly known as sterile cefoperazone sodium, and CEFOBID (cefoperazone injection), formerly known as cefoperazone sodium injection in Galaxy® plastic container (PL 2040) contain cefoperazone as cefoperazone sodium. It is a semisynthetic, broad-spectrum, cephalosporin antibiotic. Chemically, cefoperazone sodium is sodium (6 R, 7 R )-7-[( R )-2-(4-ethyl-2,3-dioxo-1-piperazinecarboxamido)-2- ( p -hydroxyphenyl)-acetamido)-3-[[(1- methyl- 1 H - tetrazol-5-yl)thio] methyl]-8-oxo-5-thia-1-azabicyclo[ 4.2.0]oct-2-ene-2-carboxylate. Its molecular formula is C 25 H 26 N 9 NaO 8 S 2 with a molecular weight of 667.65. The structural formula is given below:
 |
CEFOBID (sterile cefoperazone) contains 34 mg sodium (1.5 mEq) per gram. CEFOBID is a white powder which is freely soluble in water. The pH of a 25% (w/v) freshly reconstituted solution varies between 4.5-6.5 and the solution ranges from colorless to straw yellow depending on the concentration.
CEFOBID (sterile cefoperazone) in crystalline form is supplied in vials containing 1 g or 2 g cefoperazone as cefoperazone sodium for intravenous or intramuscular administration.
CEFOBID (sterile cefoperazone) is also supplied in Piggy Back Units for intravenous administration only.
CEFOBID (cefoperazone injection) in Galaxy® plastic container (PL 2040) is a frozen, iso-osmotic, sterile, non-pyrogenic premixed 50 mL solution containing 1 g or 2 g of cefoperazone as cefoperazone sodium. Dextrose hydrous, USP, has been added to adjust the osmolality to approximately 300 mOsmol/kg (approximately 2.3 g and 1.8 g to the 1 g and 2 g dosages, respectively). The pH may have been adjusted with sodium hydroxide and/or hydrochloric acid. After thawing to room temperature, it is intended for intravenous use only.
The Galaxy® container is fabricated from a specially designed multilayer plastic (PL 2040). Solutions are in contact with the polyethylene layer of this container and can leach out certain chemical components of the plastic in very small amounts within the expiration dating period. The suitability of the plastic has been confirmed in test in animals according to the USP biological tests for plastic containers, as well as by tissue culture toxicity studies.
High serum and bile levels of CEFOBID are attained after a single dose of the drug. Table 1 demonstrates the serum concentrations of CEFOBID in normal volunteers following either a single 15-minute constant rate intravenous infusion of 1, 2, 3 or 4 grams of the drug, or a single intramuscular injection of 1 or 2 grams of the drug.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The mean serum half-life of CEFOBID is approximately 2.0 hours, independent of the route of administration.
In vitro studies with human serum indicate that the degree of CEFOBID reversible protein binding varies with the serum concentration from 93% at 25 mcg/mL of CEFOBID to 90% at 250 mcg/mL and 82% at 500 mcg/mL.
CEFOBID achieves therapeutic concentrations in the following body tissues and fluids:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
CEFOBID is excreted mainly in the bile. Maximum bile concentrations are generally obtained between one and three hours following drug administration and exceed concurrent serum concentrations by up to 100 times. Reported biliary concentrations of CEFOBID range from 66 mcg/mL at 30 minutes to as high as 6000 mcg/mL at 3 hours after an intravenous bolus injection of 2 grams.
Following a single intramuscular or intravenous dose, the urinary recovery of CEFOBID over a 12-hour period averages 20-30%. No significant quantity of metabolites has been found in the urine. Urinary concentrations greater than 2200 mcg/mL have been obtained following a 15-minute infusion of a 2 g dose. After an IM injection of 2 g, peak urine concentrations of almost 1000 mcg/mL have been obtained, and therapeutic levels are maintained for 12 hours.
Repeated administration of CEFOBID at 12-hour intervals does not result in accumulation of the drug in normal subjects. Peak serum concentrations, areas under the curve (AUC's), and serum half-lives in patients with severe renal insufficiency are not significantly different from those in normal volunteers. In patients with hepatic dysfunction, the serum half-life is prolonged and urinary excretion is increased. In patients with combined renal and hepatic insufficiencies, CEFOBID may accumulate in the serum.
CEFOBID has been used in pediatrics, but the safety and effectiveness in children have not been established. The half-life of CEFOBID in serum is 6-10 hours in low birth-weight neonates.
CEFOBID is active in vitro against a wide range of aerobic and anaerobic, gram-positive and gram-negative pathogens. The bactericidal action of CEFOBID results from the inhibition of bacterial cell wall synthesis. CEFOBID has a high degree of stability in the presence of beta-lactamases produced by most gram-negative pathogens. CEFOBID is usually active against organisms which are resistant to other beta-lactam antibiotics because of beta-lactamase production. CEFOBID is usually active against the following organisms in vitro and in clinical infections:
Staphylococcus aureus, penicillinase and non-penicillinase-producing strains
Streptococcus pneumoniae (formerly Diplococcus pneumoniae )
Streptococcus pyogenes (Group A beta-hemolytic streptococci)
Streptococcus agalactiae (Group B beta-hemolytic streptococci)
Enterococcus ( Streptococcus faecalis, S. faecium and S. durans )
Klebsiella species (including K. pneumoniae )
Enterobacter species
Citrobacter species
Proteus mirabilis
Proteus vulgaris
Morganella morganii (formerly Proteus morganii )
Providencia rettgeri (formerly Proteus rettgeri )
Pseudomonas aeruginosa
Pseudomonas species
Some strains of Acinetobacter calcoaceticus
Gram-positive cocci (including Peptococcus and Peptostreptococcus )
Clostridium species
Other Bacteroides species
CEFOBID is also active in vitro against a wide variety of other pathogens although the clinical significance is unknown. These organisms include: Salmonella and Shigella species, Serratia liquefaciens, N. meningitidis, Bordetella pertussis, Yersinia enterocolitica, Clostridium difficile, Fusobacterium species Eubacterium species and beta-lactamase producing strains of H. influenzae and N. gonorrhoeae.
Diffusion Technique. For the disk diffusion method of susceptibility testing, a 75 mcg CEFOBID diffusion disk should be used. Organisms should be tested with the CEFOBID 75 mcg disk since CEFOBID has been shown in vitro to be active against organisms which are found to be resistant to other beta-lactam antibiotics.
Tests should be interpreted by the following criteria:
Greater than or Susceptible
equal to 21 mm
16-20 mm Moderately Susceptible
Less than or equal Resistant
to 15 mm
Quantitative procedures that require measurement of zone diameters give the most precise estimate of susceptibility. One such method which has been recommended for use with the CEFOBID 75 mcg disk is the NCCLS approved standard. (Performance Standards for Antimicrobic Disk Susceptibility Tests. Second Information Supplement Vol. 2 No. 2 pp. 49-69. Publisher--National Committee for Clinical Laboratory Standards, Villanova, Pennsylvania.)
A report of "susceptible" indicates that the infecting organism is likely to respond to CEFOBID therapy and a report of "resistant" indicates that the infecting organism is not likely to respond to therapy. A "moderately susceptible" report suggests that the infecting organism will be susceptible to CEFOBID if a higher than usual dosage is used or if the infection is confined to tissues and fluids (e.g., urine or bile) in which high antibiotic levels are attained.
Dilution Techniques. Broth or agar dilution methods may be used to determine the minimal inhibitory concentration (MIC) of CEFOBID. Serial twofold dilutions of CEFOBID should be prepared in either broth or agar. Broth should be inoculated to contain 5 × 10 5 organisms/mL and agar "spotted" with 10 4 organisms.
MIC test results should be interpreted in light of serum, tissue, and body fluid concentrations of CEFOBID. Organisms inhibited by CEFOBID at 16 mcg/mL or less are considered susceptible, while organisms with MIC's of 17-63 mcg/mL are moderately susceptible. Organisms inhibited at CEFOBID concentrations of greater than or equal to 64 mcg/mL are considered resistant, although clinical cures have been obtained in some patients infected by such organisms.
CEFOBID is indicated for the treatment of the following infections when caused by susceptible organisms:
Respiratory Tract Infections caused by S. pneumoniae, H. influenzae, S. aureus (penicillinase and non-penicillinase producing strains), S. pyogenes* (Group A beta-hemolytic streptococci), P. aeruginosa, Klebsiella pneumoniae, E. coli, Proteus mirabilis, and Enterobacter species
Peritonitis and Other Intra-abdominal Infections caused by E. coli, P. aeruginosa,* and anaerobic gram-negative bacilli (including Bacteroides fragilis ).
Bacterial Septicemia caused by S. pneumoniae, S. agalactiae*, S. aureus, Pseudomonas aeruginosa*, E. coli, Klebsiella spp., * Klebsiella pneumoniae* , Proteus species* (indole-positive and indole-negative), Clostridium spp.* and anaerobic gram-positive cocci.*
Infections of the Skin and Skin Structures caused by S. aureus (penicillinase and non-penicillinase producing strains), S. pyogenes*, and P. aeruginosa.
Pelvic Inflammatory Disease, Endometritis, and Other Infections of the Female Genital Tract caused by N. gonorrhoeae, S. epidermidis*, S. agalactiae, E. coli, Clostridium spp.,* Bacteroides species (including Bacteroides fragilis ) and anaerobic gram-positive cocci.
Urinary Tract Infections caused by Escherichia coli and Pseudomonas aeruginosa.
Enterococcal Infections: Although cefoperazone has been shown to be clinically effective in the treatment of infections caused by enterococci in cases of peritonitis and other intra-abdominal infections, infections of the skin and skin structures, pelvic inflammatory disease, endometritis and other infections of the female genital tract, and urinary tract infection,* the majority of clinical isolates of enterococci tested are not susceptible to cefoperazone but fall just at or in the intermediate zone of susceptibilty, and are moderately resistant to cefoperazone. However, in vitro susceptibility testing may not correlate directly with in vivo results. Despite this, cefoperazone therapy has resulted in clinical cures of enterococcal infections, chiefly in polymicrobial infections. Cefoperazone should be used in enterococcal infections with care and at doses that achieve satisfactory serum levels of cefoperazone.
*Efficacy of this organism in this organ system was studied in fewer than 10 infections.
Before instituting treatment with CEFOBID, appropriate specimens should be obtained for isolation of the causative organism and for determination of its susceptibility to the drug. Treatment may be started before results of susceptibility testing are available.
Synergy between CEFOBID and aminoglycosides has been demonstrated with many gram-negative bacilli. However, such enhanced activity of these combinations is not predictable. If such therapy is considered, in vitro susceptibility tests should be performed to determine the activity of the drugs in combination, and renal function should be monitored carefully. (See PRECAUTIONS , and DOSAGE AND ADMINISTRATION sections).
CEFOBID is contraindicated in patients with known allergy to the cephalosporin-class of antibiotics.
BEFORE THERAPY WITH CEFOBID IS INSTITUTED, CAREFUL INQUIRY SHOULD BE MADE TO DETERMINE WHETHER THE PATIENT HAS HAD PREVIOUS HYPERSENSITIVITY REACTIONS TO CEPHALOSPORINS, PENICILLINS OR OTHER DRUGS. THIS PRODUCT SHOULD BE GIVEN CAUTIOUSLY TO PENICILLIN-SENSITIVE PATIENTS. ANTIBIOTICS SHOULD BE ADMINISTERED WITH CAUTION TO ANY PATIENT WHO HAS DEMONSTRATED SOME FORM OF ALLERGY, PARTICULARLY TO DRUGS. SERIOUS ACUTE HYPERSENSITIVITY REACTIONS MAY REQUIRE THE USE OF SUBCUTANEOUS EPINEPHRINE AND OTHER EMERGENCY MEASURES.
PSEUDOMEMBRANOUS COLITIS HAS BEEN REPORTED WITH THE USE OF CEPHALOSPORINS (AND OTHER BROAD-SPECTRUM ANTIBIOTICS); THEREFORE, IT IS IMPORTANT TO CONSIDER ITS DIAGNOSIS IN PATIENTS WHO DEVELOP DIARRHEA IN ASSOCIATION WITH ANTIBIOTIC USE.
Treatment with broad-spectrum antibiotics alters normal flora of the colon and may permit overgrowth of clostridia. Studies indicate a toxin produced by Clostridium difficile is one primary cause of antibiotic-associated colitis. Cholestyramine and colestipol resins have been shown to bind the toxin in vitro.
Mild cases of colitis may respond to drug discontinuance alone.
Moderate to severe cases should be managed with fluid, electrolyte, and protein supplementation as indicated.
When the colitis is not relieved by drug discontinuance or when it is severe, oral vancomycin is the treatment of choice for antibiotic-associated pseudomembranous colitis produced by C. difficile. Other causes of colitis should also be considered.
Although transient elevations of the BUN and serum creatinine have been observed, CEFOBID alone does not appear to cause significant nephrotoxicity. However, concomitant administration of aminoglycosides and other cephalosporins has caused nephrotoxicity.
CEFOBID is extensively excreted in bile. The serum half-life of CEFOBID is increased 2-4 fold in patients with hepatic disease and/or biliary obstruction. In general, total daily dosage above 4 g should not be necessary in such patients. If higher dosages are used, serum concentrations should be monitored.
Because renal excretion is not the main route of elimination of CEFOBID (see ), patients with renal failure require no adjustment in dosage when usual doses are administered. When high doses of CEFOBID are used, concentrations of drug in the serum should be monitored periodically. If evidence of accumulation exists, dosage should be decreased accordingly.
The half-life of CEFOBID is reduced slightly during hemodialysis. Thus, dosing should be scheduled to follow a dialysis period. In patients with both hepatic dysfunction and significant renal disease, CEFOBID dosage should not exceed 1-2 g daily without close monitoring of serum concentrations.
As with other antibiotics, vitamin K deficiency has occurred rarely in patients treated with CEFOBID. The mechanism is most probably related to the suppression of gut flora which normally synthesize this vitamin. Those at risk include patients with a poor nutritional status, malabsorption states (e.g., cystic fibrosis), alcoholism, and patients on prolonged hyper-alimentation regimens (administered either intravenously or via a naso-gastric tube). Prothrombin time should be monitored in these patients and exogenous vitamin K administered as indicated.
A disulfiram-like reaction characterized by flushing, sweating, headache, and tachycardia has been reported when alcohol (beer, wine) was ingested within 72 hours after CEFOBID administration. Patients should be cautioned about the ingestion of alcoholic beverages following the administration of CEFOBID. A similar reaction has been reported with other cephalosporins.
Prolonged use of CEFOBID may result in the overgrowth of nonsusceptible organisms. Careful observation of the patient is essential. If superinfection occurs during therapy, appropriate measures should be taken.
CEFOBID should be prescribed with caution in individuals with a history of gastrointestinal disease, particularly colitis.
A false-positive reaction for glucose in the urine may occur with Benedict' or Fehling' solution.
Long term studies in animals have not been performed to evaluate carcinogenic potential. The maximum duration of CEFOBID animal toxicity studies is six months. In none of the in vivo or in vitro genetic toxicology studies did CEFOBID show any mutagenic potential at either the chromosomal or subchromosomal level. CEFOBID produced no impairment of fertility and had no effects on general reproductive performance or fetal development when administered subcutaneously at daily doses up to 500 to 1000 mg/kg prior to and during mating, and to pregnant female rats during gestation. These doses are 10 to 20 times the estimated usual single clinical dose. CEFOBID had adverse effects on the testes of prepubertal rats at all doses tested. Subcutaneous administration of 1000 mg/kg per day (approximately 16 times the average adult human dose) resulted in reduced testicular weight, arrested spermatogenesis, reduced germinal cell population and vacuolation of Sertoli cell cytoplasm. The severity of lesions was dose dependent in the 100 to 1000 mg/kg per day range; the low dose caused a minor decrease in spermatocytes. This effect has not been observed in adult rats. Histologically the lesions were reversible at all but the highest dosage levels. However, these studies did not evaluate subsequent development of reproductive function in the rats. The relationship of these findings to humans is unknown.
Pregnancy Category B: Reproduction studies have been performed in mice, rats, and monkeys at doses up to 10 times the human dose and have revealed no evidence of impaired fertility or harm to the fetus due to CEFOBID. There are, however, no adequate and well controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Only low concentrations of CEFOBID are excreted in human milk. Although CEFOBID passes poorly into breast milk of nursing mothers, caution should be exercised when CEFOBID is administered to a nursing woman.
Safety and effectiveness in children have not been established. For information concerning testicular changes in prepubertal rats (see Carcinogenesis, Mutagenesis, Impairment of Fertility ).
In clinical studies the following adverse effects were observed and were considered to be related to CEFOBID therapy or of uncertain etiology:
Hypersensitivity: As with all cephalosporins, hypersensitivity manifested by skin reactions (1 patient in 45), drug fever (1 in 260), or a change in Coombs' test (1 in 60) has been reported. These reactions are more likely to occur in patients with a history of allergies, particularly to penicillin.
Hematology: As with other beta-lactam antibiotics, reversible neutropenia may occur with prolonged administration. Slight decreases in neutrophil count (1 patient in 50) have been reported. Decreased hemoglobins (1 in 20) or hematocrits (1 in 20) have been reported, which is consistent with published literature on other cephalosporins. Transient eosinophilia has occurred in 1 patient in 10.
Hepatic: Of 1285 patients treated with cefoperazone in clinical trials, one patient with a history of liver disease developed significantly elevated liver function enzymes during CEFOBID therapy. Clinical signs and symptoms of nonspecific hepatitis accompanied these increases. After CEFOBID therapy was discontinued, the patient' enzymes returned to pre-treatment levels and the symptomatology resolved. As with other antibiotics that achieve high bile levels, mild transient elevations of liver function enzymes have been observed in 5-10% of the patients receiving CEFOBID therapy. The relevance of these findings, which were not accompanied by overt signs or symptoms of hepatic dysfunction, has not been established.
Gastrointestinal: Diarrhea or loose stools has been reported in 1 in 30 patients. Most of these experiences have been mild or moderate in severity and self-limiting in nature. In all cases, these symptoms responded to symptomatic therapy or ceased when cefoperazone therapy was stopped. Nausea and vomiting have been reported rarely.
Symptoms of pseudomembranous colitis can appear during or for several weeks subsequent to antibiotic therapy (see ).
Renal Function Tests: Transient elevations of the BUN (1 in 16) and serum creatinine (1 in 48) have been noted.
Local Reactions: CEFOBID is well tolerated following intramuscular administration. Occasionally, transient pain (1 in 140) may follow administration by this route. When CEFOBID is administered by intravenous infusion some patients may develop phlebitis (1 in 120) at the infusion site.
The usual adult daily dose of CEFOBID (sterile cefoperazone) is 2 to 4 grams per day administered in divided doses every 12 hours.
In severe infections or infections caused by less sensitive organisms, the total daily dose and/or frequency may be increased. Patients have been successfully treated with a total daily dosage of 6-12 grams divided into 2, 3 or 4 administrations ranging from 1.5 to 4 grams per dose.
In a pharmacokinetic study, a total daily dose of 16 grams was administered to severely immunocompromised patients by constant infusion without complications. Steady state serum concentrations were approximately 150 mcg/mL in these patients.
When treating infections caused by Streptococcus pyogenes, therapy should be continued for at least 10 days.
Solutions of CEFOBID and aminoglycoside should not be directly mixed, since there is a physical incompatibility between them. If combination therapy with CEFOBID and an aminoglycoside is contemplated (see INDICATIONS) this can be accomplished by sequential intermittent intravenous infusion provided that separate secondary intravenous tubing is used, and that the primary intravenous tubing is adequately irrigated with an approved diluent between doses. It is also suggested that CEFOBID be administered prior to the aminoglycoside. In vitro testing of the effectiveness of drug combination(s) is recommended.
The following solutions may be used for the initial reconstitution of CEFOBID (sterile cefoperazone).
|
CEFOBID (sterile cefoperazone) for intravenous or intramuscular use may be initially reconstituted with any compatible solution mentioned above in Table 1. Solutions should be allowed to stand after reconstitution to allow any foaming to dissipate to permit visual inspection for complete solubilization. Vigorous and prolonged agitation may be necessary to solubilize CEFOBID in higher concentrations (above 333 mg cefoperazone/mL). The maximum solubility of CEFOBID (sterile cefoperazone) is approximately 475 mg cefoperazone/mL of compatible diluent.
General. CEFOBID (sterile cefoperazone) concentrations between 2 mg/mL and 50 mg/mL are recommended for intravenous administration.
Preparation of Vials. Vials of CEFOBID (sterile cefoperazone) may be initially reconstituted with a minimum of 2.8 mL per gram of cefoperazone of any compatible reconstituting solution appropriate for intravenous administration listed above in Table 1. For ease of reconstitution the use of 5 mL of compatible solution per gram of CEFOBID is recommended. The entire quantity of the resulting solution should then be withdrawn for further dilution and administration using any of the following vehicles for intravenous infusion:
|
Preparation of Piggy Back Units. CEFOBID (sterile cefoperazone) in Piggy Back Units for intravenous use may be prepared by adding between 20 mL and 40 mL of any appropriate diluent listed in Table 2 per gram of cefoperazone. If 5% Dextrose and Lactated Ringer' Injection or Lactated Ringer' Injection (USP) is the chosen vehicle for administration the CEFOBID (sterile cefoperazone) should initially be reconstituted using 2.8-5 mL per gram of any compatible reconstituting solution listed in Table 1 prior to the final dilution.
The resulting intravenous solution should be administered in one of the following manners:
Intermittent Infusion: Solutions of CEFOBID should be administered over a 15-30 minute time period.
Continuous Infusion: CEFOBID can be used for continuous infusion after dilution to a final concentration of between 2 and 25 mg cefoperazone per mL.
Any suitable solution listed above may be used to prepare CEFOBID (sterile cefoperazone) for intramuscular injection. When concentrations of 250 mg/mL or more are to be administered, a lidocaine solution should be used. These solutions should be prepared using a combination of Sterile Water for Injection and 2% Lidocaine Hydrochloride Injection (USP) that approximates a 0.5% Lidocaine Hydrochloride Solution. A two-step dilution process as follows is recommended: First, add the required amount of Sterile Water for Injection and agitate until CEFOBID powder is completely dissolved. Second, add the required amount of 2% lidocaine and mix.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
CEFOBID (sterile cefoperazone) is to be stored at or below 25°C (77°F) and protected from light prior to reconstitution. After reconstitution, protection from light is not necessary.
The following parenteral diluents and approximate concentrations of CEFOBID provide stable solutions under the following conditions for the indicated time periods. (After the indicated time periods, unused portions of solutions should be discarded.)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
DIRECTIONS FOR USE OF CEFOBID ® (cefoperazone injection) IN GALAXY ® PLASTIC CONTAINER (PL 2040)
CEFOBID in Galaxy ® Container (PL 2040 Plastic) is to be administered either as a continuous or intermittent infusion.
Storage
Store in a freezer capable of maintaining a temperature of -20°C/-4°F.
Thawing of Plastic Container
Thaw frozen container at room temperature (25°C/77°F) or under refrigeration (5°C/41°F). [DO NOT FORCE THAW BY IMMERSION IN WATER BATHS OR BY MICROWAVE IRRADIATION.]
Check for minute leaks by squeezing container firmly. If leaks are detected, discard solution as sterility may be impaired.
DO NOT ADD SUPPLEMENTARY MEDICATION.
Preparation for Intravenous Administration (Use Aseptic Technique).
Caution: Do not use plastic containers in series connections. Such use could result in an embolism due to residual air being drawn from the primary container before administration of the fluid from the secondary container is complete.
CEFOBID® (sterile cefoperazone) is available in vials containing cefoperazone sodium equivalent to
1 g cefoperazone × 10 (NDC 0049-1201-83), and 2 g cefoperazone × 10 (NDC 0049-1202-83) for intramuscular and intravenous administration.
CEFOBID ® (sterile cefoperazone) is available in Piggy Back Units containing cefoperazone sodium equivalent to 1 g cefoperazone × 10 (NDC 0049-1211-83), and 2 g cefoperazone × 10 (NDC 0049-1212-83), and 10 g (NDC 0049-1219-28) Pharmacy Bulk Package for intravenous administration.
CEFOBID ® (sterile cefoperazone) is supplied as a frozen, iso-osmotic, pre-mixed solution in a single dose Galaxy ® plastic container (PL 2040) as follows:
1 g/50 mL..................... NDC 0049-1216-18
2 g/50 mL..................... NDC 0049-1215-18
Store container(s) at or below -20°C/-4°F.
See DIRECTIONS FOR USE OF CEFOBID ® (cefoperazone injection) IN GALAXY ® PLASTIC CONTAINER (PL 2040).
CEFOBID ® (cefoperazone injection) in Galaxy ® plastic container (PL 2040) is manufactured for Roerig Division of Pfizer Pharmaceuticals by Baxter Healthcare Corporation, Deerfield, IL 60015.
CEFOBID ® is a registered trademark of Pfizer Inc. Galaxy ® is a registered trademark of Baxter International Inc.
Rx only. ©1999 PFIZER INC
70-4169-00-8 Revised November 1999