 |
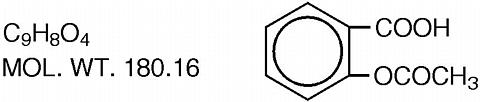
Easprin®, Aspirin Delayed-release Tablets, USP, enteric coated (E/C), contain 975 mg (15 grains) aspirin for oral administration. The enteric coating is designed to prevent the release of aspirin in the stomach and thereby reduce gastric irritation and total occult blood loss. The pharmacologic effects of aspirin include analgesia, antipyresis, antiinflammatory activity, and antirheumatic activity. The structural formula of aspirin (salicylic acid acetate) is:
 |
Microcrystalline cellulose, starch, croscarmellose sodium, silicon dioxide, talc, shellac, titanium dioxide, hydroxypropyl methylcellulose phthalate, polyvinyl acetate phthalate, cellulose acetate phthalate.
Aspirin is a salicylate that has demonstrated antiinflammatory, analgesic, antipyretic, and antirheumatic activity.
Aspirin' mode of action as an antiinflammatory and antirheumatic agent may be due to inhibition of synthesis and release of prostaglandins.
Aspirin appears to produce analgesia by virtue of both a peripheral and CNS effect. Peripherally, aspirin acts by inhibiting the synthesis and release of prostaglandins. Acting centrally, it would appear to produce analgesia at a hypothalamic site in the brain, although the mode of action is not known.
Aspirin also acts on the hypothalamus to produce antipyresis; heat dissipation is increased as a result of vasodilation and increased peripheral blood flow. Aspirin' antipyretic activity may also be related to inhibition of synthesis and release of prostaglandins.
EASPRIN Tablets are enteric coated. This coating acts to prevent the release of aspirin in the stomach but permits the tablet to dissolve with resultant absorption in the upper portion of the small intestine. This reduces any gastric irritation that may occur with uncoated aspirin but does delay the onset of action. Aspirin is rapidly hydrolyzed primarily in the liver to salicylic acid, which is conjugated with glycine (forming salicyluric acid) and glucuronic acid and excreted largely in the urine. As a result of the rapid hydrolysis, plasma concentrations of aspirin are always low and rarely exceed 20 mcg/ml at ordinary therapeutic doses. The peak salicylate level for uncoated aspirin occurs in about 2 hours, however, with enteric coated aspirin tablets this is delayed. A direct correlation between salicylate plasma levels and clinical analgesic effectiveness has not been definitely established, but effective analgesia is usually achieved at plasma levels of 15 to 30 mg per 100 ml. Effective antiinflammatory activity is usually achieved at salicylate plasma levels of 20 to 30 mg per 100 ml. There is also poor correlation between toxic symptoms and plasma salicylate concentrations, but most patients exhibit symptoms of salicylism at plasma salicylate levels of 35 mg per 100 ml. The plasma half-life for aspirin is approximately 15 minutes; that for salicylate lengthens as the dose increases. Doses of 300 to 600 mg have a half-life of 3.1 to 3.2 hours, with doses of 1 gram, the half-life is increased to 5 hours and with 2 grams it is increased to about 9 hours.
Salicylates are excreted mainly by the kidney. Studies in man indicate that salicylate is excreted in the urine as free salicylic acid (10%), salicyluric acid (75%), salicylic phenolic (10%), and acyl (5%) glucuronides and gentisic acid.
EASPRIN Tablet indicated in patients who need the higher 975 mg dose of aspirin in the long-term palliative treatment of mild to moderate pain and inflammation of arthritic and other inflammatory conditions.
EASPRIN Tablet should not be used in patients who have previously exhibited hypersensitivity to aspirin and/or nonsteroidal antiinflammatory agents.
EASPRIN Tablets should not be given to patients with a recent history of gastrointestinal bleeding or in patients with bleeding disorders (eg, hemophilia).
EASPRIN Tablets should be used with caution when anticoagulants are prescribed concurrently, for aspirin may depress the concentration of prothrombin in plasma and thereby increase bleeding time. Large doses of salicylates have a hypoglycemic action and may enhance the effect of the oral hypoglycemics. Consequently, they should not be given concomitantly; if however, this is necessary, the dosage of the hypoglycemic agent must be reduced while the salicylate is given. This hypoglycemic action may also effect the insulin requirements of diabetics.
Although salicylates in large doses are uricosuric agents, smaller amounts may decrease the uricosuric effects of probenecid, sulfinpyrazone, and phenylbutazone.
General: EASPRIN Tablets should be administered with caution to patients with asthma, nasal polyps, or nasal allergies.
In patients receiving large doses of aspirin and/or prolonged therapy, mild salicylate intoxication (salicylism) may develop that may be reversed by reduction in dosage.
Although the fecal blood loss with EASPRIN Tablets is less than that with uncoated aspirin tablets, EASPRIN Tablets should be administered with caution to patients with a history of gastric distress, ulcer, or bleeding problems. Occult gastrointestinal bleeding occurs in many patients but is not correlated with gastric distress. The amount of blood lost is usually insignificant clinically, but with prolonged administration, it may result in iron deficiency anemia.
Sodium excretion produced by spironolactone may be decreased in the presence of salicylates.
Salicylates can produce changes in thyroid function tests.
Salicylates should be used with caution in patients with severe hepatic damage, pre-existing hypoprothrombinemia or K deficiency, and in those undergoing surgery.
Hypoglycemic Agents: See .
Uricosuric Agents: Aspirin may decrease the effects of probenecid, sulfinpyrazone, and phenylbutazone.
Spironolactone: See General PRECAUTIONS above.
Alcohol Has a synergistic effect with aspirin in causing gastrointestinal bleeding.
Corticosteroids: Concomitant administration with aspirin may increase the risk of gastrointestinal ulceration.
Pyrazolone Derivatives (phenylbutazone, oxyphenbutazone, and possibly dipyrone): Concomitant administration with aspirin may increase the risk of gastrointestinal ulceration.
Nonsteroidal Antiinflammatory Agents: Aspirin is contraindicated in patients who are hypersensitive to nonsteroidal antiinflammatory agents.
Urinary Alkalinizers: Decrease aspirin effectiveness by increasing the rate of salicylate renal excretion.
Phenobarbital: Decreases aspirin effectiveness by enzyme induction.
Propranolol: May decrease aspirin' antiinflammatory action by competing for the same receptors.
Antacid EASPRIN Tablets should not be given concurrently with antacids, since an increase in the pH of the stomach may effect the enteric coating of the tablets.
Usage in Pregnancy: Aspirin does not appear to have any teratogenic effects. However, it has been reported that adverse effects were increased in the mother and fetus following chronic ingestion of aspirin. Prolonged pregnancy and labor with increased bleeding before and after delivery, as well as decreased birth weight and increased risk of stillbirth were correlated with high blood salicylate levels. Because of possible adverse effects on the neonate and the potential for increased maternal blood loss, aspirin should be avoided during the last three months of pregnancy.
Gastrointestinal: Dyspepsia, nausea, vomiting, diarrhea, gastrointestinal bleeding, and/or ulceration.
Ear: Tinnitus, vertigo, reversible hearing loss.
Hematologic: Prolongation of bleeding time, leukopenia, thrombocytopenia, purpura, decreased plasma iron concentration and shortened erythrocyte survival time.
Dermatologic and Hypersensitivity: Urticaria, angioedema, pruritus, various skin eruptions, asthma, and anaphylaxis.
Miscellaneous: Acute reversible hepatotoxicity, mental confusion, drowsiness, sweating, dizziness, headache, fever, thirst, and dimness of vision.
Overdosage of 200 to 500 mg/kg is in the fatal range. Early symptoms are CNS stimulation with vomiting, hyperpnea, hyperactivity, and possibly convulsions. This progresses quickly to depression, coma, respiratory failure, and collapse. These symptoms are accompanied by severe electrolyte disturbances.
In the treatment of salicylate overdosage, intensive supportive therapy should be instituted immediately. Plasma salicylate levels should be measured in order to determine the severity of the poisoning and to provide a guide for therapy. Emptying of the stomach should be accomplished as soon as possible with ipecac syrup unless the patient is depressed. In depressed patients use airway protected gastric lavage. Delay absorption with activated charcoal and give a saline cathartic. Proceed according to Standard Reference Procedures for Salicylate intoxication.
Usual Adult Dosage: One tablet 3 to 4 times daily.
Patients who have displayed no significant adverse effects on a long term qid regimen and who receive a total daily dosage of aspirin no greater than 3.9 grams may be considered for a bid regimen (2 EASPRIN Tablets twice daily). Patients on the bid regimen should be closely monitored for serum salicylate levels, increased incidence of CNS-related adverse effects, increased fecal blood loss, or any other signs or symptoms suggestive of significant blood loss.
If necessary, dosage may be increased until relief is obtained, but dosage should be maintained slightly below that which produces tinnitus. Plasma salicylate levels may also be helpful in determining proper dosage (see section).
EASPRIN Tablets, white, imprinted, each containing 975 mg (15 grains) aspirin are available in bottles of 100's (NDC 59417-975-71).
Storage: Store at controlled room temperature 15°-30°C (59°-86°F).
Rx only
Manufactured for:
LOTUS BIOCHEMICAL CORPORATION
Radford, VA 24143, USA
By:
Time-Cap Labs, Inc.
Farmingdale, NY 11735, USA
©Lotus Biochemical Corporation
All Rights Reserved
Rev. 07/98