 |
Lariam (mefloquine hydrochloride) is an antimalarial agent available as 250-mg tablets of mefloquine hydrochloride (equivalent to 228.0 mg of the free base) for oral administration.
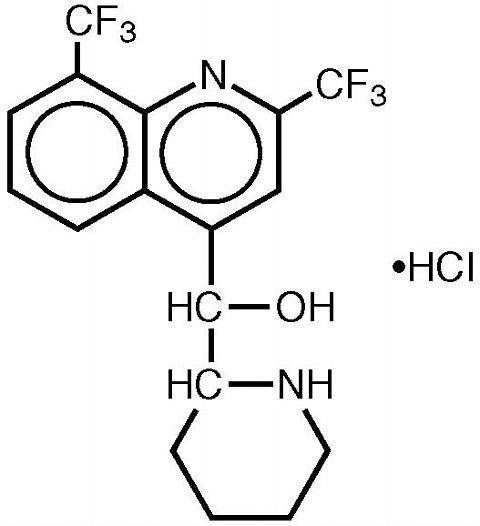
Mefloquine hydrochloride is a 4-quinolinemethanol derivative with the specific chemical name of (R*, S*)-(±)-(alpha)-2-piperidinyl-2,8-bis (trifluoromethyl)-4-quinolinemethanol hydrochloride. It is a 2-aryl substituted chemical structural analog of quinine. The drug is a white to almost white crystalline compound, slightly soluble in water.
Mefloquine hydrochloride has a calculated molecular weight of 414.78 and the following structural formula:
 |
The inactive ingredients are ammonium-calcium alginate, corn starch, crospovidone, lactose, magnesium stearate, microcrystalline cellulose, poloxamer #331, and talc.
Mefloquine is an antimalarial agent which acts as a blood schizonticide. Its exact mechanism of action is not known.
Pharmacokinetic studies of mefloquine in healthy male subjects showed that a significant lagtime occurred after drug administration, and the terminal elimination half-life varied widely (13 to 24 days) with a mean of about 3 weeks. Mefloquine is a mixture of enantiomeric molecules whose rates of release, absorption, transport, action, degradation and elimination may differ. A valid pharmacokinetic model may not exist in such a case.
Additional studies in European subjects showed slightly greater concentrations of drug for longer periods of time. The absorption half-life was 0.36 to 2 hours, and the terminal elimination half-life was 15 to 33 days. The primary metabolite was identified and its concentrations were found to surpass the concentrations of mefloquine.
Multiple-dose kinetic studies confirmed the long elimination half-lives previously observed. The mean metabolite to mefloquine ratio measured at steady-state was found to range between 2.3 and 8.6.
The total clearance of the drug, which is essentially all hepatic, is approximately 30 mL/min. The volume of distribution, approximately 20 L/kg, indicates extensive distribution. The drug is highly bound (98%) to plasma proteins and concentrated in blood erythrocytes, the target cells in malaria, at a relatively constant erythrocyte-to-plasma concentration ratio of about 2.
The pharmacokinetics of mefloquine in patients with compromised renal function and compromised hepatic function have not been studied.
In vitro and in vivo studies showed no hemolysis associated with glucose-6-phosphate dehydrogenase deficiency (see ANIMAL TOXICOLOGY ).
Microbiology Strains of Plasmodium falciparum resistant to mefloquine have been reported.
Treatment of Acute Malaria Infections: Lariam is indicated for the treatment of mild to moderate acute malaria caused by mefloquine-susceptible strains of P. falciparum (both chloroquine-susceptible and resistant strains) or by Plasmodium vivax . There are insufficient clinical data to document the effect of mefloquine in malaria caused by P. ovale or P. malariae .
Note: Patients with acute P. vivax malaria, treated with Lariam, are at high risk of relapse because Lariam does not eliminate exoerythrocytic (hepatic phase) parasites. To avoid relapse, after initial treatment of the acute infection with Lariam, patients should subsequently be treated with an 8-aminoquinoline (eg, primaquine).
Prevention of Malaria: Lariam is indicated for the prophylaxis of P. falciparum and P. vivax malaria infections, including prophylaxis of chloroquine-resistant strains of P. falciparum .
Use of Lariam is contraindicated in patients with a known hypersensitivity to mefloquine or related compounds (eg, quinine and quinidine). Lariam should not be prescribed for prophylaxis in patients with active depression or with a history of psychosis or convulsions.
In case of life-threatening, serious or overwhelming malaria infections due to P. falciparum , patients should be treated with an intravenous antimalarial drug. Following completion of intravenous treatment, Lariam may be given to complete the course of therapy.
Data on the use of halofantrine subsequent to administration of Lariam suggests a significant, potentially fatal prolongation of the QTc interval of the ECG. Therefore, halofantrine must not be given simultaneously with or subsequent to Lariam. No data are available on the use of Lariam after halofantrine (see PRECAUTIONS: Drug Interactions ).
Concomitant administration of Lariam and quinine or quinidine may produce electrocardiographic abnormalities.
Concomitant administration of Lariam and quinine or chloroquine may increase the risk of convulsions.
General: In patients with epilepsy, Lariam may increase the risk of convulsions. The drug should therefore be prescribed only for curative treatment in such patients and only if there are compelling medical reasons for its use (see PRECAUTIONS: Drug Interactions ).
Caution should be exercised with regard to activities requiring alertness and fine motor coordination such as driving, piloting aircraft and operating machinery, as dizziness, a loss of balance, or other disorders of the central or peripheral nervous system have been reported during and following the use of Lariam. These effects may occur after therapy is discontinued due to the long half-life of the drug. During prophylactic use, if signs of acute anxiety, depression, restlessness or confusion occur, these may be considered prodromal to a more serious event. In these cases, the drug must be discontinued. Lariam should be used with caution in patients with psychiatric disturbances because mefloquine use has been associated with emotional disturbances (see ADVERSE REACTIONS ).
In patients with impaired liver function the elimination of mefloquine may be prolonged, leading to higher plasma levels.
This drug has been administered for longer than 1 year. If the drug is to be administered for a prolonged period, periodic evaluations including liver function tests should be performed. Although retinal abnormalities seen in humans with long-term chloroquine use have not been observed with mefloquine use, long-term feeding of mefloquine to rats resulted in dose-related ocular lesions (retinal degeneration, retinal edema and lenticular opacity at 12.5 mg/kg/day and higher) (see ANIMAL TOXICOLOGY ). Therefore, periodic ophthalmic examinations are recommended.
Parenteral studies in animals show that mefloquine, a myocardial depressant, possesses 20% of the antifibrillatory action of quinidine and produces 50% of the increase in the PR interval reported with quinine. The effect of mefloquine on the compromised cardiovascular system has not been evaluated. However, transitory and clinically silent ECG alterations have been reported during the use of mefloquine. Alterations included sinus bradycardia, sinus arrhythmia, first degree AV-block, prolongation of the QTc interval and abnormal T waves (see also cardiovascular effects under PRECAUTIONS: Drug Interactions and ADVERSE REACTIONS ). The benefits of Lariam therapy should be weighed against the possibility of adverse effects in patients with cardiac disease.
Laboratory Tests: Periodic evaluation of hepatic function should be performed during prolonged prophylaxis.
Information for Patients: Patients should be advised:
Drug Interactions: Drug-drug interactions with Lariam have not been explored in detail. There is one report of cardiopulmonary arrest, with full recovery, in a patient who was taking a beta blocker (propranolol) (see PRECAUTIONS: General ). The effects of mefloquine on the compromised cardiovascular system have not been evaluated. The benefits of Lariam therapy should be weighed against the possibility of adverse effects in patients with cardiac disease.
Because of the danger of a potentially fatal prolongation of the QTc interval, halofantrine should not be given simultaneously with or subsequent to Lariam (see ).
Concomitant administration of Lariam and other related compounds (eg, quinine, quinidine and chloroquine) may produce electrocardiographic abnormalities and increase the risk of convulsions (see CONTRAINDICATIONS ). If these drugs are to be used in the initial treatment of severe malaria, Lariam administration should be delayed at least 12 hours after the last dose. There is evidence that the use of halofantrine after mefloquine causes a significant lengthening of the QTc interval. Clinically significant QTc prolongation has not been found with mefloquine alone.
This appears to be the only clinically relevant interaction of this kind with Lariam, although theoretically, coadministration of other drugs known to alter cardiac conduction (eg, anti-arrhythmic or beta-adrenergic blocking agents, calcium channel blockers, antihistamines or H1-blocking agents, tricyclic antidepressants and phenothiazines) might also contribute to a prolongation of the QTc interval. There are no data that conclusively establish whether the concomitant administration of mefloquine and the above listed agents has an effect on cardiac function.
In patients taking an anticonvulsant (eg, valproic acid, carbamazepine, phenobarbital or phenytoin), the concomitant use of Lariam may reduce seizure control by lowering the plasma levels of the anticonvulsant. Therefore, patients concurrently taking antiseizure medication and Lariam should have the blood level of their antiseizure medication monitored and the dosage adjusted appropriately (see PRECAUTIONS: General ).
When Lariam is taken concurrently with oral live typhoid vaccines, attenuation of immunization cannot be excluded. Vaccinations with attenuated live bacteria should therefore be completed at least 3 days before the first dose of Lariam.
No other drug interactions are known. Nevertheless, the effects of Lariam on travelers receiving comedication, particularly those on anticoagulants or antidiabetics, should be checked before departure.
In clinical trials, the concomitant administration of sulfadoxine and pyrimethamine did not alter the adverse reaction profile.
Carcinogenesis, Mutagenesis, Impairment of Fertility: Carcinogenesis: The carcinogenic potential of mefloquine was studied in rats and mice in 2-year feeding studies at doses of up to 30 mg/kg/day. No treatment-related increases in tumors of any type were noted.
Mutagenesis The mutagenic potential of mefloquine was studied in a variety of assay systems including: Ames test, a host-mediated assay in mice, fluctuation tests and a mouse micronucleus assay. Several of these assays were performed with and without prior metabolic activation. In no instance was evidence obtained for the mutagenicity of mefloquine.
Impairment of Fertility: Fertility studies in rats at doses of 5, 20, and 50 mg/kg/day of mefloquine have demonstrated adverse effects on fertility in the male at the high dose of 50 mg/kg/day, and in the female at doses of 20 and 50 mg/kg/day. Histopathological lesions were noted in the epididymides from male rats at doses of 20 and 50 mg/kg/day. Administration of 250 mg/week of mefloquine (base) in adult males for 22 weeks failed to reveal any deleterious effects on human spermatozoa.
Pregnancy: Teratogenic Effects. Pregnancy Category C. Mefloquine has been demonstrated to be teratogenic in rats and mice at a dose of 100 mg/kg/day. In rabbits, a high dose of 160 mg/kg/day was embryotoxic and teratogenic, and a dose of 80 mg/kg/day was teratogenic but not embryotoxic. There are no adequate and well-controlled studies in pregnant women. However, clinical experience with Lariam has not revealed an embryotoxic or teratogenic effect. Mefloquine should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Women of childbearing potential who are traveling to areas where malaria is endemic should be warned against becoming pregnant. Women of childbearing potential should also be advised to practice contraception during malaria prophylaxis with Lariam.
Nursing Mothers: Mefloquine is excreted in human milk. Based on a study in a few subjects, low concentrations (3% to 4%) of mefloquine were excreted in human milk following a dose equivalent to 250 mg of the free base. Because of the potential for serious adverse reactions in nursing infants from mefloquine, a decision should be made whether to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use: Use of Lariam to treat acute, uncomplicated P. falciparum malaria in pediatric patients is supported by evidence from adequate and well-controlled studies of Lariam in adults with additional data from published open-label and comparative trials using Lariam to treat malaria caused by P. falciparum in patients younger than 16 years of age. The safety and effectiveness of Lariam for the treatment of malaria in pediatric patients below the age of 6 months have not been established.
In several studies, the administration of Lariam for the treatment of malaria was associated with early vomiting in pediatric patients. Early vomiting was cited in some reports as a possible cause of treatment failure. If a second dose is not tolerated, the patient should be monitored closely and alternative malaria treatment considered if improvement is not observed within a reasonable period of time (see DOSAGE AND ADMINISTRATION ).
Clinical At the doses used for treatment of acute malaria infections, the symptoms possibly attributable to drug administration cannot be distinguished from those symptoms usually attributable to the disease itself.
Among subjects who received mefloquine for prophylaxis of malaria, the most frequently observed adverse experience was vomiting (3%). Dizziness, syncope, extrasystoles and other complaints affecting less than 1% were also reported.
Among subjects who received mefloquine for treatment, the most frequently observed adverse experiences included: dizziness, myalgia, nausea, fever, headache, vomiting, chills, diarrhea, skin rash, abdominal pain, fatigue, loss of appetite, and tinnitus. Those side effects occurring in less than 1% included bradycardia, hair loss, emotional problems, pruritus, asthenia, transient emotional disturbances and telogen effluvium (loss of resting hair). Seizures have also been reported.
Two serious adverse reactions were cardiopulmonary arrest in one patient shortly after ingesting a single prophylactic dose of mefloquine while concomitantly using propranolol (see PRECAUTIONS ), and encephalopathy of unknown etiology during prophylactic mefloquine administration. The relationship of encephalopathy to drug administration could not be clearly established.
Postmarketing: Postmarketing surveillance indicates that the same adverse experiences are reported during prophylaxis, as well as acute treatment.
The most common adverse reactions to Lariam prophylaxis, namely nausea, vomiting, and dizziness, are generally mild and may decrease with prolonged use, in spite of increasing plasma drug levels. In a large study of tourists receiving various prophylactic antimalarials, the rate of subjects reporting adverse events on Lariam was similar to that of tourists on chloroquine.
The most frequently reported adverse events are nausea, vomiting, dizziness or vertigo, loss of balance, headache, somnolence, sleep disorders (insomnia, abnormal dreams), loose stools or diarrhea, and abdominal pain.
Less frequently reported adverse events:
Central and peripheral nervous system: convulsions, depression, hallucinations, psychotic or paranoid reactions, anxiety, agitation, aggression, confusion, forgetfulness, hearing impairment, restlessness, sensory and motor neuropathies (including paresthesia), tinnitus and vestibular disorders, visual disturbances. Suicidal ideation has also rarely been reported, but no relationship to drug administration has been established.
Cardiovascular system: circulatory disturbances (hypotension, hypertension, flushing, syncope), tachycardia or palpitations, bradycardia, irregular pulse, extrasystoles and other transient cardiac conduction alterations.
Skin rash, exanthema, erythema, urticaria, pruritus, hair loss, sweating.
Musculoskeletal system: muscle weakness, muscle cramps, myalgia, arthralgia.
General symptoms: asthenia, malaise, fatigue, fever, chills, loss of appetite.
Isolated cases of erythema multiforme, Stevens-Johnson syndrome, AV-block, and encephalopathy, have been reported.
Laboratory The most frequently observed laboratory alterations which could be possibly attributable to drug administration were decreased hematocrit, transient elevation of transaminases, leukopenia and thrombocytopenia. These alterations were observed in patients with acute malaria who received treatment doses of the drug and were attributed to the disease itself.
During prophylactic administration of mefloquine to indigenous populations in malaria-endemic areas, the following occasional alterations in laboratory values were observed: transient elevation of transaminases, leukocytosis or thrombocytopenia.
Because of the long half-life of mefloquine, adverse reactions to Lariam may occur or persist up to several weeks after the last dose.
In cases of overdosage with Lariam, the symptoms mentioned under ADVERSE REACTIONS may be more pronounced. The following procedure is recommended in case of overdosage: Induce vomiting or perform gastric lavage, as appropriate. Monitor cardiac function (if possible by ECG) and neurologic and psychiatric status for at least 24 hours. Provide symptomatic and intensive supportive treatment as required, particularly for cardiovascular disturbances. Treat vomiting or diarrhea with standard fluid therapy.
DOSAGE AND ADMINISTRATION (see )
Adult Patients: Treatment of mild to moderate malaria in adults caused by P. vivax or mefloquine-susceptible strains of P. falciparum: Five tablets (1250 mg) mefloquine hydrochloride to be given as a single oral dose. The drug should not be taken on an empty stomach and should be administered with at least 8 oz (240 mL) of water.
If a full treatment course has been administered without clinical cure, alternative treatment should be given. Similarly, if previous prophylaxis with mefloquine has failed, Lariam should not be used for curative treatment.
Note: Patients with acute P. vivax malaria, treated with Lariam, are at high risk of relapse because Lariam does not eliminate exoerythrocytic (hepatic phase) parasites. To avoid relapse after initial treatment of the acute infection with Lariam, patients should subsequently be treated with an 8-aminoquinoline (eg, primaquine).
Malaria prophylaxis: One 250 mg Lariam tablet once weekly.
Prophylactic drug administration should begin 1 week before departure to an endemic area. Subsequent weekly doses should always be taken on the same day of the week. To reduce the risk of malaria after leaving an endemic area, prophylaxis should be continued for 4 additional weeks. Tablets should not be taken on an empty stomach and should be administered with at least 8 oz (240 mL) of water.
In certain cases, eg, when a traveler is taking other medication, it may be desirable to start prophylaxis 2 to 3 weeks prior to departure, in order to ensure that the combination of drugs is well tolerated.
Pediatric Patients: Treatment of mild to moderate malaria in pediatric patients caused by mefloquine-susceptible strains of P. falciparum: 20 to 25 mg/kg for non-immune patients. Splitting the total curative dose into 2 doses taken 6 to 8 hours apart may reduce the occurrence or severity of adverse effects. Experience with Lariam in infants less than 3 months old or weighing less than 5 kg is limited. The drug should not be taken on an empty stomach and should be administered with ample water. For very young patients, the dose may be crushed, mixed with water or sugar water and may be administered via an oral syringe.
If a full-treatment course has been administered without clinical cure, alternative treatment should be given. Similarly, if previous prophylaxis with mefloquine has failed, Lariam should not be used for curative treatment.
In pediatric patients, the administration of Lariam for the treatment of malaria has been associated with early vomiting. In some cases, early vomiting has been cited as a possible cause of treatment failure (see PRECAUTIONS ). If a significant loss of drug product is observed or suspected because of vomiting, a second full dose of Lariam should be administered to patients who vomit less than 30 minutes after receiving the drug. If vomiting occurs 30 to 60 minutes after a dose, an additional half-dose should be given. If vomiting recurs, the patient should be monitored closely and alternative malaria treatment considered if improvement is not observed within a reasonable period of time.
The safety and effectiveness of Lariam to treat malaria in pediatric patients below the age of 6 months have not been established.
Malaria Prophylaxis: The following doses have been extrapolated from the recommended adult dose. Neither the pharmacokinetics, nor the clinical efficacy of these doses have been determined in children owing to the difficulty of acquiring this information in pediatric subjects. The recommended prophylactic dose of Lariam is 3 to 5 mg/kg once weekly. One 250 mg Lariam tablet should be taken once weekly in pediatric patients weighing over 45 kg. In pediatric patients weighing less than 45 kg, the weekly dose decreases in proportion to body weight:
>30 to 45 kg: ¾ tablet
>20 to 30 kg: ½ tablet
up to 20 kg: ¼ tablet
Experience with Lariam in infants less than 3 months old or weighing less than 5 kg is limited.
Lariam is available as scored, white, round tablets, containing 250 mg of mefloquine hydrochloride in unit-dose packages of 25 (NDC 0004-0172-02). Imprint on tablets: LARIAM 250 ROCHE
Tablets should be stored at 15° to 30°C (59° to 86°F).
 |
Ocular lesions were observed in rats fed mefloquine daily for 2 years. All surviving rats given 30 mg/kg/day had ocular lesions in both eyes characterized by retinal degeneration, opacity of the lens, and retinal edema. Similar but less severe lesions were observed in 80% of female and 22% of male rats fed 12.5 mg/kg/day for 2 years. At doses of 5 mg/kg/day, only corneal lesions were observed. They occurred in 9% of rats studied.
Manufactured by
F. HOFFMANN-LA ROCHE LTD
Basel, Switzerland
Distributed by
Roche Pharmaceuticals
Revised: August 1999