 |
CAUTION: Federal law prohibits dispensing without prescription.
Each milliliter of Melanex® Topical Solution contains 30 mg of hydroquinone in a hydroalcoholic base of purified water, SD Alcohol 40 (45%), Laureth-4, Isopropyl Alcohol (4%), Propylene Glycol, Ascorbic Acid.
 |
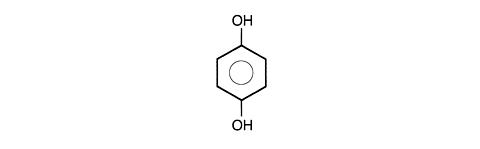
C 6 H 6 O 2 110.11
1,4 DIHYDROXYBENZENE
It has been suggested the primary action of hydroquinone is directed at tyrosinase. 1 The selective inhibition of the enzyme affects melanogenesis in the melanocytes resulting in cessation of melanin formation and subsequent reduction in pigmentation. Additional studies indicate hydroquinone acts on the essential subcellular metabolic processes of melanocytes with resultant cytolysis, i.e., non-enzyme-mediated depigmentation. 2
Melanex® is indicated in the temporary depigmentation of hyperpigmented skin conditions such as chloasma, melasma, freckles, senile lentigines, and other forms of melanin hyperpigmentation.
Apply to affected areas twice daily, in the morning and before bedtime. During the day, an effective broad spectrum sunscreen like Neutrogena® Sunblock SPF 15 or SPF 30 should be used and unnecessary solar exposure avoided, or protective clothing should be worn to cover the treated area in order to prevent repigmentation from occurring.
Melanex® is contraindicated in persons who have shown hypersensitivity to hydroquinone or any of the other ingredients. The safety of topical treatment with hydroquinone during pregnancy has not been established.
Concurrent use of Melanex® with peroxide products may result in transient dark staining of skin areas so treated. This is due to the oxidation of hydroquinone by the peroxide. This transient staining can be removed by discontinuing concurrent usage and normal soap cleansing.
Hydroquinone preparations may produce skin irritation in susceptible individuals and have a slight potential to produce allergic response. Therefore, the physician should use appropriate caution. If rash or irritation develops, discontinue use and consult physician. Do not use on children under 12 years.
If no improvement is seen after two months of treatment, use of product should be discontinued. Avoid contact with eyes. In case of accidental contact, patient should rinse eyes thoroughly with water and contact physician. A bitter taste and anesthetic effect may occur if applied to lips. Keep out of reach of children. Use of Melanex® in paranasal and infraorbital areas increases the chance of irritation (see ADVERSE REACTIONS ).
The following have been reported: dryness and fissuring of the paranasal and infraorbital areas, erythema, and stinging. Hydroquinone has been known to produce irritation and sensitization in susceptible individuals.
HOW SUPPLIED: 1 fl. oz. (30 ML) bottle with plastic rod and Appliderm® Applicator unit.
NOTE : Slight darkening of the Melanex® solution is normal and will not affect potency. See expiration date on bottle.
 |
Store at room temperature or below. Avoid excessive heat.
|
(1)
|
JIMBOW K., OBATHA H., PATHAK M., FITZPATRICK T.B. Mechanism and Depigmentation of Hydroquinone, Journal of Investigative Dermatology 1974, 62:436-449.
|
|
(2)
|
op. cit.
|
For additional information please call:
Neutrogena Technical Department toll-free (800) 421-6857; in California call (800) 649-1150.
NDC 10812-930-01
Distributed by
Neutrogena Dermatologics
5760 W. 96th St.
Los Angeles, CA 90045
 |