 |
WARNINGNOVANTRONE® (mitoxantrone for injection concentrate) should be administered under the supervision of a physician experienced in the use of cancer chemotherapeutic agents. Except for the treatment of acute nonlymphocytic leukemia, NOVANTRONE® therapy generally should not be given to patients with baseline neutrophil counts of less than 1,500 cells/mm 3 . In order to monitor the occurrence of bone marrow suppression, primarily neutropenia, which may be severe and result in infection, it is recommended that frequent peripheral blood cell counts be performed on all patients receiving NOVANTRONE®. |
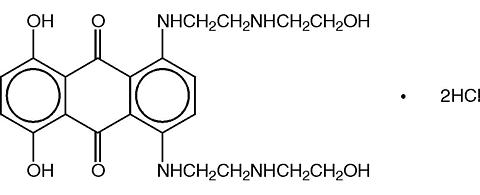
NOVANTRONE® (mitoxantrone hydrochloride) is a synthetic antineoplastic anthracenedione for intravenous use. The molecular formula is C 22 H 28 N 4 O 6 ·2HCl and the molecular weight is 517.41. It is supplied as a concentrate which MUST BE DILUTED PRIOR TO INJECTION. The concentrate is a sterile, nonpyrogenic, dark blue aqueous solution containing mitoxantrone hydrochloride equivalent to 2 mg/mL mitoxantrone free base, with sodium chloride (0.80% w/v), sodium acetate (0.005% w/v), and acetic acid (0.046% w/v) as inactive ingredients. The solution has a pH of 3.0 to 4.5 and contains 0.14 mEq of sodium per mL. The product does not contain preservatives. The chemical name is 1,4-dihydroxy-5,8-bis[[2-[(2-hydroxyethyl) amino]ethyl]amino]-9,10-anthracenedione dihydrochloride and the structural formula is:
 |
Although its mechanism of action is not fully elucidated, mitoxantrone is a DNA-reactive agent. It has a cytocidal effect on both proliferating and nonproliferating cultured human cells, suggesting lack of cell cycle phase specificity.
of mitoxantrone in patients following a single intravenous administration of NOVANTRONE can be characterized by a three-compartment model. The mean alpha half-life of mitoxantrone is 6 to 12 minutes, the mean beta half-life is 1.1 to 3.1 hours and the mean gamma (terminal or elimination) half-life is 23 to 215 hours (median approximately 75 hours). Pharmacokinetic studies have not been performed in humans receiving multiple daily dosing. Distribution to tissues is extensive: steady-state volume of distribution exceeds 1,000 L/m2. Tissue concentrations of mitoxantrone appear to exceed those in the blood during the terminal elimination phase. In the monkey, distribution to brain, spinal cord, eye, and spinal fluid is low.
In patients administered 15-90 mg/m 2 of NOVANTRONE intravenously, there is a linear relationship between dose and the area under the concentration-time curve.
Mitoxantrone is 78% bound to plasma proteins in the observed concentration range of 26-455 ng/mL. This binding is independent of concentration and is not affected by the presence of phenytoin, doxorubicin, methotrexate, prednisone, prednisolone, heparin, or aspirin.
Metabolism and Elimination: Metabolism and elimination of mitoxantrone following NOVANTRONE administration are not well characterized. Eleven percent or less of mitoxantrone is recovered in the urine, and 25% or less is recovered in the feces, within five days after drug administration. Of the material recovered in the urine, 65% is unchanged drug. The remaining 35% is comprised primarily of a mono- and a dicarboxylic acid derivative and their glucuronide conjugates. These carboxylic acid metabolites are not DNA-reactive/cytocidal, and their route of formation is unknown.
Gender The effect of gender on mitoxantrone pharmacokinetics is unknown.
Geriatric Mitoxantrone pharmacokinetics in the elderly are unknown.
Pediatric Mitoxantrone pharmacokinetics in the pediatric population are unknown.
Race: The effect of race on mitoxantrone pharmacokinetics is unknown.
Renal Impairment: Mitoxantrone pharmacokinetics in patients with renal impairment are unknown.
Hepatic Impairment: Mitoxantrone clearance is reduced by hepatic impairment. Patients with severe hepatic dysfunction (bilirubin greater than 3.4 mg/dL) have an AUC more than 3-fold that of patients with normal hepatic function receiving the same dose. For patients with hepatic impairment, there is at present no laboratory measurement that allows for dose adjustment recommendations.
Drug Interactions: Pharmacokinetic studies of the interaction of NOVANTRONE with concomitantly administered medications have not been performed. The interaction of mitoxantrone with the human P450 system has not been investigated.
Advanced Hormone-Refractory Prostate Cancer
A multicenter phase 2 trial of NOVANTRONE and low-dose prednisone (N + P) was conducted in 27 symptomatic patients with hormone-refractory prostate cancer. Using NPCP (National Prostate Cancer Project) criteria for disease response, there was one partial responder and 12 patients with stable disease. However, nine patients or 33% achieved a palliative response defined on the basis of reduction in analgesic use or pain intensity.
These findings led to the initiation of a randomized multicenter trial (CCI-NOV22) comparing the effectiveness of (N + P) to low-dose prednisone alone (P). Eligible patients were required to have metastatic or locally advanced disease that had progressed on standard hormonal therapy, a castrate serum testosterone level, and at least mild pain at study entry. NOVANTRONE was administered at a dose of 12 mg/m 2 by short IV infusion every three weeks. Prednisone was administered orally at a dose of 5 mg twice a day. Patients randomized to the prednisone arm were crossed over to the N + P arm if they progressed or if they were not improved after a minimum of six weeks of therapy with prednisone alone.
A total of 161 patients were randomized, 80 to the N + P arm and 81 to the P arm. The median NOVANTRONE dose administered was 12 mg/m 2 per cycle. The median cumulative NOVANTRONE dose administered was 73 mg/m 2 (range of 12 to 212 mg/m 2 ).
A primary palliative response (defined as a 2-point decrease in pain intensity in a 6-point pain scale, associated with stable analgesic use, and lasting a minimum of 6 weeks) was achieved in 29% of patients randomized to N + P compared to 12% of patients randomized to P alone (p = 0.011). Two responders left the study after meeting primary response criterion for two consecutive cycles. For the purposes of this analysis, these two patients were assigned a response duration of zero days. A secondary palliative response was defined as a 50% or greater decrease in analgesic use, associated with stable pain intensity, and lasting a minimum of 6 weeks. An overall palliative response (defined as primary plus secondary responses) was achieved in 38% of patients randomized to N + P compared to 21% of patients randomized to P (p = 0.025).
The median duration of primary palliative response for patients randomized to N + P was 7.6 months compared to 2.1 months for patients randomized to P alone (p = 0.0009). The median duration of overall palliative response for patients randomized to N + P was 5.6 months compared to 1.9 months for patients randomized to P alone (p = 0.0004).
Time to progression was defined as a 1-point increase in pain intensity, or a >25% increase in analgesic use, or evidence of disease progression on radiographic studies, or requirement for radiotherapy. The median time to progression for all patients randomized to N + P was 4.4 months compared to 2.3 months for all patients randomized to P alone (p = 0.0001). Median time to death was 11.3 months for all patients on the N + P arm compared to 10.8 months for all patients on P alone (p = 0.2324).
Forty-eight patients on the P arm crossed over to receive N + P. Of these, thirty patients had progressed on P, while 18 had stable disease on P. The median cycle of crossover was 5 cycles (range of 2 to 16 cycles). Time trends for pain intensity prior to crossover were significantly worse for patients who crossed over than for those who remained on P alone (p = 0.012). Nine patients (19%) demonstrated a palliative response on N + P after crossover. The median time to death for patients who crossed over to N + P was 12.7 months.
The clinical significance of a fall in prostate specific antigen (PSA) concentrations after chemotherapy is unclear. On the CCI-NOV22 trial, a PSA fall of 50% or greater for two consecutive follow-up assessments after baseline was reported in 33% of all patients randomized to the N+P arm and 9% of all patients randomized to the P arm. These findings should be interpreted with caution since PSA responses were not defined prospectively. A number of patients were inevaluable for response, and there was an imbalance between treatment arms in the numbers of evaluable patients. In addition, PSA reduction did not correlate precisely with palliative response, the primary efficacy endpoint of this study. For example, among the 26 evaluable patients randomized to the N+P arm who had a >/=50% reduction in PSA, only 13 had a primary palliative response. Also, among 42 evaluable patients on this arm who did not have this reduction in PSA, 8 nonetheless had a primary palliative response.
Investigators at Cancer and Leukemia Group B (CALGB) conducted a phase III comparative trial of NOVANTRONE plus hydrocortisone (N + H) versus hydrocortisone alone (H) in patients with hormone-refractory prostate cancer (CALGB 9182). Eligible patients were required to have metastatic disease that had progressed despite at least one hormonal therapy. Progression at study entry was defined on the basis of progressive symptoms, increases in measurable or osseous disease, or rising PSA levels. NOVANTRONE was administered intravenously at a dose of 14 mg/m 2 every 21 days and hydrocortisone was administered orally at a daily dose of 40 mg. A total of 242 subjects were randomized, 119 to the N + H arm and 123 to the H arm. There were no differences in survival between the two arms, with a median of 11.1 months in the N + H arm, and 12 months in the H arm (p = 0.3298).
Using NPCP criteria for response, partial responses were achieved in 10 patients (8.4%) randomized to the N + H arm compared with 2 patients (1.6%) randomized to the H arm (p = 0.018). The median time to progression, defined by NPCP criteria, for patients randomized to the N + H arm was 7.3 months compared to 4.1 months for patients randomized to H alone (p = 0.0654).
Approximately 60% of patients on each arm required analgesics at baseline. Analgesic use was measured in this study using a 5-point scale. The best percent change from baseline in mean analgesic use was -17% for 61 patients with available data on the N + H arm, compared with +17% for 61 patients on H alone (p = 0.014). A time trend analysis for analgesic use in individual patients also showed a trend favoring the N + H arm over H alone but was not statistically significant.
Pain intensity was measured using the Symptom Distress Scale (SDS) Pain Item 2 (a 5-point scale). The best percent change from baseline in mean pain intensity was -14% for 37 patients with available data on the N + H arm, compared with +8% for 38 patients on H alone (p = 0.057). A time trend analysis for pain intensity in individual patients showed no difference between treatment arms.
In two large randomized multicenter trials, remission induction therapy for acute nonlymphocytic leukemia (ANLL) with NOVANTRONE 12 mg/m 2 daily for 3 days as a 10-minute intravenous infusion and cytarabine 100 mg/m 2 for 7 days given as a continuous 24-hour infusion was compared with daunorubicin 45 mg/m 2 daily by intravenous infusion for 3 days plus the same dose and schedule of cytarabine used with NOVANTRONE. Patients who had an incomplete antileukemic response received a second induction course in which NOVANTRONE or daunorubicin was administered for 2 days and cytarabine for 5 days using the same daily dosage schedule. Response rates and median survival information for both the U.S. and international multicenter trials are given in the following table:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
In these studies, two consolidation courses were administered to complete responders on each arm. Consolidation therapy consisted of the same drug and daily dosage used for remission induction, but only 5 days of cytarabine and 2 days of NOVANTRONE or daunorubicin were given. The first consolidation course was administered 6 weeks after the start of the final induction course if the patient achieved a complete remission. The second consolidation course was generally administered 4 weeks later. Full hematologic recovery was necessary for patients to receive consolidation therapy. For the U.S. trial, median granulocyte nadirs for patients receiving NOVANTRONE + cytarabine for consolidation courses 1 and 2 were 10/mm 3 for both courses, and for those patients receiving daunorubicin + cytarabine nadirs were 170/mm 3 and 260/mm 3 , respectively. Median platelet nadirs for patients who received NOVANTRONE + cytarabine for consolidation courses 1 and 2 were 17,000/mm 3 and 14,000/mm 3 , respectively, and were 33,000/mm 3 and 22,000/mm 3 in courses 1 and 2 for those patients who received daunorubicin + cytarabine. The benefit of consolidation therapy in ANLL patients who achieve a complete remission remains controversial. However, in the only well-controlled prospective, randomized multicenter trials with NOVANTRONE in ANLL, consolidation therapy was given to all patients who achieved a complete remission. During consolidation in the U.S. study, two myelosuppression-related deaths occurred on the NOVANTRONE arm and one on the daunorubicin arm. However, in the international study there were eight deaths on the NOVANTRONE arm during consolidation which were related to the myelosuppression and none on the daunorubicin arm where less myelosuppression occurred.
NOVANTRONE in combination with corticosteroids is indicated as initial chemotherapy for the treatment of patients with pain related to advanced hormone-refractory prostate cancer.
NOVANTRONE in combination with other approved drug(s) is indicated in the initial therapy of acute nonlymphocytic leukemia (ANLL) in adults. This category includes myelogenous, promyelocytic, monocytic, and erythroid acute leukemias.
NOVANTRONE is contraindicated in patients who have demonstrated prior hypersensitivity to it.
WHEN NOVANTRONE IS USED IN DOSES INDICATED FOR THE TREATMENT OF LEUKEMIA, SEVERE MYELOSUPPRESSION WILL OCCUR. THEREFORE, IT IS RECOMMENDED THAT NOVANTRONE BE ADMINISTERED ONLY BY PHYSICIANS EXPERIENCED IN THE CHEMOTHERAPY OF THIS DISEASE. LABORATORY AND SUPPORTIVE SERVICES MUST BE AVAILABLE FOR HEMATOLOGIC AND CHEMISTRY MONITORING AND ADJUNCTIVE THERAPIES, INCLUDING ANTIBIOTICS. BLOOD AND BLOOD PRODUCTS MUST BE AVAILABLE TO SUPPORT PATIENTS DURING THE EXPECTED PERIOD OF MEDULLARY HYPOPLASIA AND SEVERE MYELOSUPPRESSION. PARTICULAR CARE SHOULD BE GIVEN TO ASSURING FULL HEMATOLOGIC RECOVERY BEFORE UNDERTAKING CONSOLIDATION THERAPY (IF THIS TREATMENT IS USED) AND PATIENTS SHOULD BE MONITORED CLOSELY DURING THIS PHASE.
Patients with preexisting myelosuppression as the result of prior drug therapy should not receive NOVANTRONE unless it is felt that the possible benefit from such treatment warrants the risk of further medullary suppression.
The safety of NOVANTRONE in patients with hepatic insufficiency is not established. (See section
Safety for use by routes other than intravenous administration has not been established.
Pregnancy - NOVANTRONE may cause fetal harm when administered to a pregnant woman. In treated rats, at doses of >/=0.1 mg/kg (0.05 fold the recommended human dose on a mg/m 2 basis) low fetal birth weight and retarded development of the fetal kidney were seen in greater frequency. In treated rabbits, an increased incidence of premature delivery was observed at doses >/=0.01 mg/kg (0.01 fold the recommended human dose on a mg/m 2 basis). NOVANTRONE was not teratogenic in rabbits. There are no adequate and well-controlled studies in pregnant women. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. Women of childbearing potential should be advised to avoid becoming pregnant.
Topoisomerase II inhibitors, including NOVANTRONE, in combination with other antineoplastic agents, have been associated with the development of acute leukemia.
Because of the possible danger of cardiac effects in patients previously treated with daunorubicin or doxorubicin, the benefit-to-risk ratio of NOVANTRONE therapy in such patients should be determined before starting therapy.
General - Functional cardiac changes including decreases in left ventricular ejection fraction (LVEF) and irreversible congestive heart failure can occur with NOVANTRONE. Cardiac toxicity may be more common in patients with prior treatment with anthracyclines, prior mediastinal radiotherapy, or with preexisting cardiovascular disease. Such patients should have regular cardiac monitoring of LVEF from the initiation of therapy. In investigational trials of intermittent single doses in other tumor types, patients who received up to the cumulative dose of 140 mg/m 2 had a cumulative 2.6% probability of clinical congestive heart failure. The overall cumulative probability rate of moderate or serious decreases in LVEF at this dose was 13% in comparative trials.
Leukemia - Acute congestive heart failure may occasionally occur in patients treated with NOVANTRONE for ANLL. In first-line comparative trials of NOVANTRONE + cytarabine vs daunorubicin + cytarabine in adult patients with previously untreated ANLL, therapy was associated with congestive heart failure in 6.5% of patients on each arm. A causal relationship between drug therapy and cardiac effects is difficult to establish in this setting since myocardial function is frequently depressed by the anemia, fever and infection, and hemorrhage which often accompany the underlying disease.
Hormone-Refractory Prostate Cancer - Functional cardiac changes such as decreases in LVEF and congestive heart failure may occur in patients with hormone-refractory prostate cancer treated with NOVANTRONE. In a randomized comparative trial of NOVANTRONE plus low-dose prednisone vs low-dose prednisone, 7 of 128 patients (5.5%) treated with NOVANTRONE had a cardiac event defined as any decrease in LVEF below the normal range, congestive heart failure (n = 3), or myocardial ischemia. Two patients had a prior history of cardiac disease. The total NOVANTRONE dose administered to patients with cardiac effects ranged from >48 to 212 mg/m 2 .
Among 112 patients evaluable for safety on the NOVANTRONE + hydrocortisone arm of the CALGB trial, 18 patients (19%) had a reduction in cardiac function, 5 patients (5%) had cardiac ischemia, and 2 patients (2%) experienced pulmonary edema. The range of total NOVANTRONE doses administered to these patients is not available.
General: Therapy with NOVANTRONE should be accompanied by close and frequent monitoring of hematologic and chemical laboratory parameters, as well as frequent patient observation.
Systemic infections should be treated concomitantly with or just prior to commencing therapy with NOVANTRONE.
Information for Patients: NOVANTRONE may impart a blue-green color to the urine for 24 hours after administration, and patients should be advised to expect this during therapy. Bluish discoloration of the sclera may also occur. Patients should be advised of the signs and symptoms of myelosuppression.
Laboratory Tests: Serial complete blood counts and liver function tests are necessary for appropriate dose adjustments. (See DOSAGE AND ADMINISTRATION section.)
In leukemia treatment, hyperuricemia may occur as a result of rapid lysis of tumor cells by NOVANTRONE. Serum uric acid levels should be monitored and hypouricemic therapy instituted prior to the initiation of antileukemic therapy.
Carcinogenesis : Intravenous treatment of rats and mice, once every 21 days for 24 months, with NOVANTRONE resulted in an increased incidence of fibroma and external auditory canal tumors in rats at a dose of 0.03 mg/kg (0.02 fold the recommended human dose, on a mg/m 2 basis), and hepatocellular adenoma in male mice at a dose of 0.1 mg/kg (0.03 fold the recommended human dose, on a mg/m 2 basis
Mutagenesis : NOVANTRONE produced a clastogenic effect in vivo (rat bone marrow metaphase analysis) and in vitro (induced DNA damage in primary rat hepatocytes and SCE in CHO cells), and is mutagenic in bacterial (Ames/Salmonella and E.Coli) and mammalian (L5178Y TK+/-mouse lymphoma) test systems.
Impairment of Fertility : Daily treatment of male rats (71 days prior to, and during the mating period, and until confirmation of pregnancy in females) and female rats (15 days prior to, and during the mating period) with NOVANTRONE IV doses up to 0.03 mg/kg (0.02 fold the recommended human dose, on a mg/m 2 basis) had no effects on fertility.
Drug Interactions: There is no evidence for drug-drug interactions when NOVANTRONE is administered with corticosteroids.
Pregnancy Pregnancy Category D: (See section
Nursing Mothers: NOVANTRONE is excreted in human milk and significant concentrations (18 ng/mL) have been reported for 28 days after the last administration. Because of the potential for serious adverse reactions in infants from NOVANTRONE, breast feeding should be discontinued before starting treatment.
Pediatric Use: Safety and effectiveness in pediatric patients have not been established.
Leukemia - NOVANTRONE® has been studied in approximately 600 patients with ANLL. The table below represents the adverse reaction experience in the large U.S. comparative study of mitoxantrone + cytarabine vs daunorubicin + cytarabine. Experience in the large international study was similar. A much wider experience in a variety of other tumor types revealed no additional important reactions other than cardiomyopathy. (See section.) It should be appreciated that the listed adverse reaction categories include overlapping clinical symptoms related to the same condition, e.g., dyspnea, cough and pneumonia. In addition, the listed adverse reactions cannot all necessarily be attributed to chemotherapy as it is often impossible to distinguish effects of the drug and effects of the underlying disease. It is clear, however, that the combination of NOVANTRONE + cytarabine was responsible for nausea and vomiting, alopecia, mucositis/stomatitis, and myelosuppression.
The following table summarizes adverse reactions occurring in patients treated with NOVANTRONE + cytarabine in comparison with those who received daunorubicin + cytarabine for therapy of ANLL in a large multicenter randomized prospective U.S. trial. Adverse reactions are presented as major categories and selected examples of clinically significant subcategories.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Hormone-Refractory Prostate Cancer - Detailed safety information is available for a total of 353 patients with hormone-refractory prostate cancer treated with NOVANTRONE, including 274 patients who received NOVANTRONE in combination with corticosteroids.
The following table summarizes adverse reactions of all grades occurring in >/=5% of patients in Trial CCI-NOV22.
|
No non-hematologic adverse events of Grade 3/4 were seen in >5% of patients.
The next table summarizes adverse events of all grades occurring in >/=5% of patients in Trial CALGB 9182.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Allergic Reaction: Hypotension, urticaria, dyspnea, and rashes have been reported occasionally.
Cutaneous Extravasation at the infusion site has been reported, which may result in erythema, swelling, pain, burning, and/or blue discoloration of the skin. Extravasation can result in tissue necrosis with resultant need for debridement and skin grafting. Phlebitis has also been reported at the site of infusion.
Hematologic: Topoisomerase II inhibitors, including NOVANTRONE in combination with other antineoplastic agents, have been associated with the development of acute leukemia.
Leukemia - Myelosuppression is rapid in onset and is consistent with the requirement to produce significant marrow hypoplasia in order to achieve a response in acute leukemia. The incidences of infection and bleeding seen in the U.S. trial are consistent with those reported for other standard induction regimens.
Hormone-refractory prostate cancer - In a randomized study where dose escalation was required for nadir neutrophil counts greater than 1000/mm 3 , Grade 4 neutropenia (ANC < 500 /mm 3 ) was observed in 54% of patients treated with NOVANTRONE + low-dose prednisone. In a separate randomized trial where patients were treated with 14 mg/m 2 , Grade 4 neutropenia in 23% of patients treated with NOVANTRONE + hydrocortisone was observed. Neutropenic fever/infection occurred in 11% and 10% of patients receiving NOVANTRONE + corticosteroids, respectively, on the two trials. Platelets < 50,000/mm 3 were noted in 4% and 3% of patients receiving NOVANTRONE + corticosteroids on these trials, and there was one patient death on NOVANTRONE + hydrocortisone due to intracranial hemorrhage after a fall.
Gastrointestinal Nausea and vomiting occurred acutely in most patients and may have contributed to reports of dehydration, but were generally mild to moderate and could be controlled through the use of antiemetics. Stomatitis/mucositis occurred within 1 week of therapy.
Cardiovascular: Congestive heart failure, tachycardia, EKG changes including arrhythmias, chest pain, and asymptomatic decreases in left ventricular ejection fraction have occurred. (See section
Pulmonary Interstitial pneumonitis has been reported in cancer patients receiving combination chemotherapy that included NOVANTRONE.
There is no known specific antidote for NOVANTRONE. Accidental overdoses have been reported. Four patients receiving 140 - 180 mg/m 2 as a single bolus injection died as a result of severe leukopenia with infection. Hematologic support and antimicrobial therapy may be required during prolonged periods of medullary hypoplasia.
Although patients with severe renal failure have not been studied, NOVANTRONE is extensively tissue bound and it is unlikely that the therapeutic effect or toxicity would be mitigated by peritoneal or hemodialysis.
Hormone-Refractory Prostate Cancer: Based on data from two Phase III comparative trials of NOVANTRONE plus corticosteroids versus corticosteroids alone, the recommended dosage of NOVANTRONE is 12 to 14 mg/m 2 given as a short intravenous infusion every 21 days.
Combination Initial Therapy for ANLL in Adults: For induction, the recommended dosage is 12 mg/m 2 of NOVANTRONE daily on days 1-3 given as an intravenous infusion, and 100 mg/m 2 of cytarabine for 7 days given as a continuous 24-hour infusion on days 1-7.
Most complete remissions will occur following the initial course of induction therapy. In the event of an incomplete antileukemic response, a second induction course may be given. NOVANTRONE should be given for 2 days and cytarabine for 5 days using the same daily dosage levels.
If severe or life-threatening nonhematologic toxicity is observed during the first induction course, the second induction course should be withheld until toxicity clears.
Consolidation therapy which was used in 2 large randomized multicenter trials consisted of NOVANTRONE, 12 mg/m 2 given by intravenous infusion daily on days 1 and 2 and cytarabine, 100 mg/m 2 for 5 days given as a continuous 24-hour infusion on days 1-5. The first course was given approximately 6 weeks after the final induction course, the second was generally administered 4 weeks after the first. Severe myelosuppression occurred. (See section
Hepatic Impairment: For patients with hepatic impairment, there is at present no laboratory measurement that allows for dose adjustment recommendations. (See , Special Populations: Hepatic Impairment )
Preparation and Administration Precautions: NOVANTRONE CONCENTRATE MUST BE DILUTED PRIOR TO USE.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
The dose of NOVANTRONE should be diluted to at least 50 mL with either 0.9% Sodium Chloride Injection (USP) or 5% Dextrose Injection (USP). NOVANTRONE may be further diluted into Dextrose 5% in Water, Normal Saline or Dextrose 5% with Normal Saline and used immediately. DO NOT FREEZE.
NOVANTRONE should not be mixed in the same infusion as heparin since a precipitate may form. Because specific compatibility data are not available, it is recommended that NOVANTRONE not be mixed in the same infusion with other drugs. The diluted solution should be introduced slowly into the tubing as a freely running intravenous infusion of 0.9% Sodium Chloride Injection (USP) or 5% Dextrose Injection (USP) over a period of not less than 3 minutes. Unused infusion solutions should be discarded immediately in an appropriate fashion. In the case of multidose use, after penetration of the stopper, the remaining portion of the undiluted NOVANTRONE concentrate should be stored not longer than 7 days between 15°-25° C (59°-77° F) or 14 days under refrigeration. DO NOT FREEZE. CONTAINS NO PRESERVATIVE.
If extravasation occurs, the administration should be stopped immediately and restarted in another vein. The nonvesicant properties of NOVANTRONE minimize the possibility of severe local reactions following extravasation. However, care should be taken to avoid extravasation at the infusion site and to avoid contact of NOVANTRONE with the skin, mucous membranes or eyes.
Skin accidentally exposed to NOVANTRONE should be rinsed copiously with warm water and if the eyes are involved, standard irrigation techniques should be used immediately. The use of goggles, gloves, and protective gowns is recommended during preparation and administration of the drug.
Procedures for proper handling and disposal of anticancer drugs should be considered. Several guidelines on this subject have been published. 1-7 There is no general agreement that all of the procedures recommended in the guidelines are necessary or appropriate.
NOVANTRONE® (mitoxantrone for injection concentrate) is a sterile aqueous solution containing mitoxantrone hydrochloride at a concentration equivalent to 2 mg mitoxantrone free base per mL supplied in vials for multidose use as follows:
NDC 58406-640-03 - 10 mL/multidose vial (20 mg)
NDC 58406-640-05 - 12.5 mL/multidose vial (25 mg)
NDC 58406-640-07 - 15 mL/multidose vial (30 mg)
NOVANTRONE® (mitoxantrone for injection concentrate) should be stored between 15°-25°C (59°-77°F). DO NOT FREEZE.
Manufactured for IMMUNEX CORPORATION, Seattle, WA 98101
by LEDERLE PARENTERALS, INC., Carolina, Puerto Rico 00987
Rev 0166-08 CI 6102-1
Revised 03/2000 ©2000 Immunex Corporation