 |
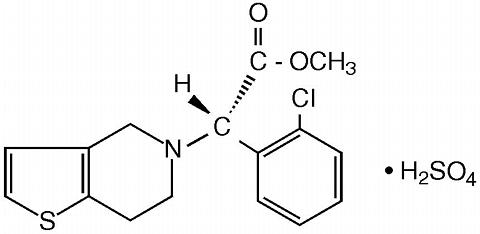
PLAVIX (clopidogrel bisulfate) is an inhibitor of ADP-induced platelet aggregation acting by direct inhibition of adenosine diphosphate (ADP) binding to its receptor and of the subsequent ADP-mediated activation of the glycoprotein GPIIb/IIIa complex. Chemically it is methyl (+)-( S )-(alpha)-(2-chlorophenyl)-6,7-dihydrothieno[3,2-c]pyridine-5(4 H )-acetate sulfate (1:1). The empirical formula of clopidogrel bisulfate is C 16 H 16 Cl NO 2 S·H 2 SO 4 and its molecular weight is 419.9.
The structural formula is as follows:
 |
Clopidogrel bisulfate is a white to off-white powder. It is practically insoluble in water at neutral pH but freely soluble at pH 1. It also dissolves freely in methanol, dissolves sparingly in methylene chloride, and is practically insoluble in ethyl ether. It has a specific optical rotation of about +56°.
PLAVIX for oral administration is provided as pink, round, biconvex, debossed film-coated tablets containing 97.875 mg of clopidogrel bisulfate which is the molar equivalent of 75 mg of clopidogrel base.
Each tablet contains anhydrous lactose, hydrogenated castor oil, microcrystalline cellulose, polyethylene glycol 6000 and pregelatinized starch as inactive ingredients. The pink film coating contains ferric oxide (red), hydroxypropyl methylcellulose 2910, polyethylene glycol 6000 and titanium dioxide. The tablets are polished with Carnauba wax.
Clopidogrel is an inhibitor of platelet aggregation. A variety of drugs that inhibit platelet function have been shown to decrease morbid events in people with established atherosclerotic cardiovascular disease as evidenced by stroke or transient ischemic attacks, myocardial infarction, or need for bypass or angioplasty. This indicates that platelets participate in the initiation and/or evolution of these events and that inhibiting them can reduce the event rate.
Clopidogrel selectively inhibits the binding of adenosine diphosphate (ADP) to its platelet receptor and the subsequent ADP-mediated activation of the glycoprotein GPIIb/IIIa complex, thereby inhibiting platelet aggregation. Biotransformation of clopidogrel is necessary to produce inhibition of platelet aggregation, but an active metabolite responsible for the activity of the drug has not been isolated. Clopidogrel also inhibits platelet aggregation induced by agonists other than ADP by blocking the amplification of platelet activation by released ADP. Clopidogrel does not inhibit phosphodiesterase activity.
Clopidogrel acts by irreversibly modifying the platelet ADP receptor. Consequently, platelets exposed to clopidogrel are affected for the remainder of their lifespan.
Dose dependent inhibition of platelet aggregation can be seen 2 hours after single oral doses of PLAVIX. Repeated doses of 75 mg PLAVIX per day inhibit ADP-induced platelet aggregation on the first day, and inhibition reaches steady state between Day 3 and Day 7. At steady state, the average inhibition level observed with a dose of 75 mg PLAVIX per day was between 40% and 60%. Platelet aggregation and bleeding time gradually return to baseline values after treatment is discontinued, generally in about 5 days.
After repeated 75-mg oral doses of clopidogrel (base), plasma concentrations of the parent compound, which has no platelet inhibiting effect, are very low and are generally below the quantification limit (0.00025 mg/L) beyond 2 hours after dosing. Clopidogrel is extensively metabolized by the liver. The main circulating metabolite is the carboxylic acid derivative, and it too has no effect on platelet aggregation. It represents about 85% of the circulating drug-related compounds in plasma.
Following an oral dose of 14 C-labeled clopidogrel in humans, approximately 50% was excreted in the urine and approximately 46% in the feces in the 5 days after dosing. The elimination half-life of the main circulating metabolite was 8 hours after single and repeated administration. Covalent binding to platelets accounted for 2% of radiolabel with a half-life of 11 days.
Effect of Food: Administration of PLAVIX (clopidogrel bisulfate) with meals did not significantly modify the bioavailability of clopidogrel as assessed by the pharmacokinetics of the main circulating metabolite.
Absorption and Distribution: Clopidogrel is rapidly absorbed after oral administration of repeated doses of 75 mg clopidogrel (base), with peak plasma levels ([cong ]3 mg/L) of the main circulating metabolite occurring approximately 1 hour after dosing. The pharmacokinetics of the main circulating metabolite are linear (plasma concentrations increased in proportion to dose) in the dose range of 50 to 150 mg of clopidogrel. Absorption is at least 50% based on urinary excretion of clopidogrel-related metabolites.
Clopidogrel and the main circulating metabolite bind reversibly in vitro to human plasma proteins (98% and 94%, respectively). The binding is nonsaturable in vitro up to a concentration of 100 µg/mL.
Metabolism and Elimination: In vitro and in vivo , clopidogrel undergoes rapid hydrolysis into its carboxylic acid derivative. In plasma and urine, the glucuronide of the carboxylic acid derivative is also observed.
Geriatric Patients: Plasma concentrations of the main circulating metabolite are significantly higher in elderly (>/=75 years) compared to young healthy volunteers but these higher plasma levels were not associated with differences in platelet aggregation and bleeding time. No dosage adjustment is needed for the elderly.
Renally Impaired Patients: After repeated doses of 75 mg PLAVIX per day, plasma levels of the main circulating metabolite were lower in patients with severe renal impairment (creatinine clearance from 5 to 15 mL/min) compared to subjects with moderate renal impairment (creatinine clearance 30 to 60 mL/min) or healthy subjects. Although inhibition of ADP-induced platelet aggregation was lower (25%) than that observed in healthy volunteers, the prolongation of bleeding time was similar to healthy volunteers receiving 75 mg of PLAVIX per day. No dosage adjustment is needed in renally impaired patients.
Gender: No significant difference was observed in the plasma levels of the main circulating metabolite between males and females. In a small study comparing men and women, less inhibition of ADP-induced platelet aggregation was observed in women, but there was no difference in prolongation of bleeding time. In the large, controlled clinical study (Clopidogrel vs. Aspirin in Patients at Risk of Ischemic Events; CAPRIE), the incidence of clinical outcome events, other adverse clinical events, and abnormal clinical laboratory parameters was similar in men and women.
Race: Pharmacokinetic differences due to race have not been studied.
The clinical evidence for the efficacy of PLAVIX is derived from the CAPRIE (Clopidogrel vs. Aspirin in Patients at Risk of Ischemic Events) trial. This was a 19,185-patient, 304-center, international, randomized, double-blind, parallel-group study comparing PLAVIX (75 mg daily) to aspirin (325 mg daily). The patients randomized had: 1) recent histories of myocardial infarction (within 35 days); 2) recent histories of ischemic stroke (within 6 months) with at least a week of residual neurological signs; or 3) objectively established peripheral arterial disease. Patients received randomized treatment for an average of 1.6 years (maximum of 3 years).
The trial' primary outcome was the time to first occurrence of new ischemic stroke (fatal or not), new myocardial infarction (fatal or not), or other vascular death. Deaths not easily attributable to nonvascular causes were all classified as vascular.
Outcome Events of the Primary Analysis
|
As shown in the table, PLAVIX (clopidogrel bisulfate) was associated with a lower incidence of outcome events of every kind. The overall risk reduction (9.78% vs. 10.64%) was 8.7%, P=0.045. Similar results were obtained when all-cause mortality and all-cause strokes were counted instead of vascular mortality and ischemic strokes (risk reduction 6.9%). In patients who survived an on-study stroke or myocardial infarction, the incidence of subsequent events was again lower in the PLAVIX group.
The curves showing the overall event rate are shown in the figure. The event curves separated early and continued to diverge over the 3-year follow-up period.
 |
Although the statistical significance favoring PLAVIX over aspirin was marginal (P=0.045), and represents the result of a single trial that has not been replicated, the comparator drug, aspirin, is itself effective (vs. placebo) in reducing cardiovascular events in patients with recent myocardial infarction or stroke. Thus, the difference between PLAVIX and placebo, although not measured directly, is substantial.
The CAPRIE trial included a population that was randomized on the basis of 3 entry criteria. The efficacy of PLAVIX relative to aspirin was heterogeneous across these randomized subgroups (P=0.043). It is not clear whether this difference is real or a chance occurrence. Although the CAPRIE trial was not designed to evaluate the relative benefit of PLAVIX over aspirin in the individual patient subgroups, the benefit appeared to be strongest in patients who were enrolled because of peripheral vascular disease (especially those who also had a history of myocardial infarction) and weaker in stroke patients. In patients who were enrolled in the trial on the sole basis of a recent myocardial infarction, PLAVIX was not numerically superior to aspirin.
In the meta-analyses of studies of aspirin vs. placebo in patients similar to those in CAPRIE, aspirin was associated with a reduced incidence of atherothrombotic events. There was a suggestion of heterogeneity in these studies too, with the effect strongest in patients with a history of myocardial infarction, weaker in patients with a history of stroke, and not discernible in patients with a history of peripheral vascular disease. With respect to the inferred comparison of PLAVIX to placebo, there is no indication of heterogeneity.
PLAVIX (clopidogrel bisulfate) is indicated for the reduction of atherosclerotic events (myocardial infarction, stroke, and vascular death) in patients with atherosclerosis documented by recent stroke, recent myocardial infarction, or established peripheral arterial disease.
The use of PLAVIX is contraindicated in the following conditions:
Thrombotic thrombocytopenic purpura (TTP): TTP has been reported rarely following use of PLAVIX, sometimes after a short exposure (<2 weeks). TTP is a serious condition requiring prompt treatment. It is characterized by thrombocytopenia, microangiopathic hemolytic anemia (schistocytes [fragmented RBCs] seen on peripheral smear), neurological findings, renal dysfunction, and fever. TTP was not seen during clopidogrel's clinical trials, which included over 11,300 clopidogrel-treated patients. In world-wide postmarketing experience, however, TTP has been reported at a rate of about four cases per million patients exposed, or about 11 cases per million patient-years. The background rate is thought to be about four cases per million person-years.
As with other anti-platelet agents, PLAVIX should be used with caution in patients who may be at risk of increased bleeding from trauma, surgery, or other pathological conditions. If a patient is to undergo elective surgery and an antiplatelet effect is not desired, PLAVIX should be discontinued 7 days prior to surgery.
GI Bleeding: PLAVIX prolongs the bleeding time. In CAPRIE, PLAVIX was associated with a rate of gastrointestinal bleeding of 2.0%, vs. 2.7% on aspirin. PLAVIX should be used with caution in patients who have lesions with a propensity to bleed (such as ulcers). Drugs that might induce such lesions (such as aspirin and other nonsteroidal anti-inflammatory drugs [NSAIDs]) should be used with caution in patients taking PLAVIX.
Use in Hepatically Impaired Patients: Experience is limited in patients with severe hepatic disease, who may have bleeding diatheses. PLAVIX should be used with caution in this population.
Patients should be told that it may take them longer than usual to stop bleeding when they take PLAVIX, and that they should report any unusual bleeding to their physician. Patients should inform physicians and dentists that they are taking PLAVIX before any surgery is scheduled and before any new drug is taken.
Study of specific drug interactions yielded the following results:
Aspirin: Aspirin did not modify the clopidogrel-mediated inhibition of ADP-induced platelet aggregation. Concomitant administration of 500 mg of aspirin twice a day for 1 day did not significantly increase the prolongation of bleeding time induced by PLAVIX. PLAVIX potentiated the effect of aspirin on collagen-induced platelet aggregation. The safety of chronic concomitant administration of aspirin and PLAVIX has not been established.
Heparin: In a study in healthy volunteers, PLAVIX did not necessitate modification of the heparin dose or alter the effect of heparin on coagulation. Coadministration of heparin had no effect on inhibition of platelet aggregation induced by PLAVIX. The safety of this combination has not been established, however, and concomitant use should be undertaken with caution.
Nonsteroidal Anti-Inflammatory Drugs (NSAIDs): In healthy volunteers receiving naproxen, concomitant administration of PLAVIX was associated with increased occult gastrointestinal blood loss. NSAIDs and PLAVIX should be coadministered with caution.
Warfarin: The safety of the coadministration of PLAVIX with warfarin has not been established. Consequently, concomitant administration of these two agents should be undertaken with caution. (See Precautions - General ).
Other Concomitant Therapy: No clinically significant pharmacodynamic interactions were observed when PLAVIX was coadministered with atenolol, nifedipine , or both atenolol and nifedipine. The pharmacodynamic activity of PLAVIX was also not significantly influenced by the coadministration of phenobarbital, cimetidine or estrogen .
The pharmacokinetics of digoxin or theophylline were not modified by the coadministration of PLAVIX (clopidogrel bisulfate).
At high concentrations in vitro , clopidogrel inhibits P 450 (2C9). Accordingly, PLAVIX may interfere with the metabolism of phenytoin, tamoxifen, tolbutamide, warfarin, torsemide, fluvastatin, and many nonsteroidal anti-inflammatory agents , but there are no data with which to predict the magnitude of these interactions. Caution should be used when any of these drugs is coadministered with PLAVIX.
In addition to the above specific interaction studies, patients entered into CAPRIE received a variety of concomitant medications including diuretics, beta-blocking agents, angiotensin converting enzyme inhibitors, calcium antagonists, cholesterol lowering agents, coronary vasodilators, antidiabetic agents, antiepileptic agents and hormone replacement therapy without evidence of clinically significant adverse interactions.
None known.
There was no evidence of tumorigenicity when clopidogrel was administered for 78 weeks to mice and 104 weeks to rats at dosages up to 77 mg/kg per day, which afforded plasma exposures >25 times that in humans at the recommended daily dose of 75 mg.
Clopidogrel was not genotoxic in four in vitro tests (Ames test, DNA-repair test in rat hepatocytes, gene mutation assay in Chinese hamster fibroblasts, and metaphase chromosome analysis of human lymphocytes) and in one in vivo test (micronucleus test by oral route in mice).
Clopidogrel was found to have no effect on fertility of male and female rats at oral doses up to 400 mg/kg per day (52 times the recommended human dose on a mg/m 2 basis
Pregnancy Category B. Reproduction studies performed in rats and rabbits at doses up to 500 and 300 mg/kg/day (respectively, 65 and 78 times the recommended daily human dose on a mg/m 2 basis), revealed no evidence of impaired fertility or fetotoxicity due to clopidogrel. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of a human response, PLAVIX should be used during pregnancy only if clearly needed.
Studies in rats have shown that clopidogrel and/or its metabolites are excreted in the milk. It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the nursing woman.
Safety and effectiveness in the pediatric population have not been established.
PLAVIX has been evaluated for safety in more than 11,300 patients, including over 7,000 patients treated for 1 year or more. The overall tolerability of PLAVIX was similar to that of aspirin regardless of age, gender and race, with an approximately equal incidence (13%) of patients withdrawing from treatment because of adverse reactions. The clinically important adverse events observed in CAPRIE are discussed below.
Hemorrhagic: In patients receiving PLAVIX in CAPRIE, gastrointestinal hemorrhage occurred at a rate of 2.0%, and required hospitalization in 0.7%. In patients receiving aspirin, the corresponding rates were 2.7% and 1.1%, respectively. The incidence of intracranial hemorrhage was 0.4% for PLAVIX compared to 0.5% for aspirin.
Neutropenia/agranulocytosis: Ticlopidine, a drug chemically similar to PLAVIX, is associated with a 0.8% rate of severe neutropenia (less than 450 neutrophils/µL). Patients in CAPRIE (see Clinical Trials ) were intensively monitored for neutropenia. Severe neutropenia was observed in six patients, four on PLAVIX and two on aspirin. Two of the 9599 patients who received PLAVIX and none of the 9586 patients who received aspirin had neutrophil counts of zero.
One of the four PLAVIX patients was receiving cytotoxic chemotherapy, and another recovered and returned to the trial after only temporarily interrupting treatment with PLAVIX.
Although the risk of myelotoxicity with PLAVIX thus appears to be quite low, this possibility should be considerd when a patient receiving PLAVIX demonstrates fever or other sign of infection.
Gastrointestinal: Overall, the incidence of gastrointestinal events (e.g. abdominal pain, dyspepsia, gastritis and constipation) in patients receiving PLAVIX (clopidogrel bisulfate) was 27.1%, compared to 29.8% in those receiving aspirin.
The incidence of peptic, gastric or duodenal ulcers was 0.7% for PLAVIX and 1.2% for aspirin.
Cases of diarrhea were reported in 4.5% of patients in the PLAVIX group compared to 3.4% in the aspirin group. However, these were rarely severe (PLAVIX=0.2% and aspirin=0.1%).
The incidence of patients withdrawing from treatment because of gastrointestinal adverse reactions was 3.2% for PLAVIX and 4.0% for aspirin.
Rash and Other Skin Disorders: The incidence of skin and appendage disorders in patients receiving PLAVIX was 15.8% (0.7% serious); the corresponding rate in aspirin patients was 13.1% (0.5% serious).
The overall incidence of patients withdrawing from treatment because of skin and appendage disorders adverse reactions was 1.5% for PLAVIX and 0.8% for aspirin.
Adverse events occurring in >/=2.5% of patients on PLAVIX in the CAPRIE controlled clinical trial are shown below regardless of relationship to PLAVIX. The median duration of therapy was 20 months, with a maximum of 3 years.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Other adverse experiences of potential importance occurring in 1% to 2.5% of patients receiving PLAVIX (clopidogrel bisulfate) in the CAPRIE controlled clinical trial are listed below regardless of relationship to PLAVIX. In general, the incidence of these events was similar in the aspirin-treated group.
Autonomic Nervous System Disorders: Syncope, Palpitation. Body as a Whole - general disorders: Asthenia, Hernia. Cardiovascular disorders: Cardiac failure. Central and peripheral nervous system disorders: Cramps legs, Hypoaesthesia, Neuralgia, Paraesthesia, Vertigo. Gastrointestinal system disorders: Constipation, Vomiting. Heart rate and rhythm disorders: Fibrillation atrial. Liver and biliary system disorders: Hepatic enzymes increased. Metabolic and nutritional disorders: Gout, hyperuricemia, non-protein nitrogen (NPN) increased. Musculo-skeletal system disorders: Arthritis, Arthrosis. Platelet, bleeding & clotting disorders: GI hemorrhage, hematoma, platelets decreased. Psychiatric disorders: Anxiety, Insomnia. Red blood cell disorders: Anemia Respiratory system disorders: Pneumonia, Sinusitis. Skin and appendage disorders: Eczema, Skin ulceration. Urinary system disorders: Cystitis Vision disorders: Cataract, Conjunctivitis.
Other potentially serious adverse events which may be of clinical interest but were rarely reported (<1%) in patients who received PLAVIX are listed below regardless of relationship to PLAVIX. In general, the incidence of these events was similar in the aspirin group.
Body as a whole: Allergic reaction, necrosis ischemic. Cardiovascular disorders: Edema generalized. Gastrointestinal system disorders: Gastric ulcer perforated, gastritis hemorrhagic, upper GI ulcer hemorrhagic. Liver and Biliary system disorders: Bilirubinemia, hepatitis infectious, liver fatty. Platelet, bleeding and clotting disorders: hemarthrosis, hematuria, hemoptysis, hemorrhage intracranial, hemorrhage retroperitoneal, hemorrhage of operative wound, ocular hemorrhage, pulmonary hemorrhage, purpura allergic, thrombocytopenia. Red blood cell disorders: Anemia aplastic, anemia hypochromic. Reproductive disorders, female: Menorrhagia. Respiratory system disorders: Hemothorax Skin and appendage disorders: Bullous eruption, rash erythematous, rash maculopapular, urticaria. White cell and reticuloendothelial system disorders: Agranulocytosis, granulocytopenia, leukemia, leukopenia, neutrophils decreased.
The following events have been reported spontaneously from worldwide postmarketing experience: very rare cases of hypersensitivity reactions including angioedema, bronchospasms, and anaphylactoid reactions. Suspected thrombotic thrombocytopenic purpura (TTP) has been reported as part of the world-wide postmarketing experience, see .
One case of deliberate overdosage with PLAVIX was reported in the large, controlled clinical study. A 34-year-old woman took a single 1,050-mg dose of PLAVIX (equivalent to 14 standard 75-mg tablets). There were no associated adverse events. No special therapy was instituted, and she recovered without sequelae.
No adverse events were reported after single oral administration of 600 mg (equivalent to 8 standard 75-mg tablets) of PLAVIX in healthy volunteers. The bleeding time was prolonged by a factor of 1.7, which is similar to that typically observed with the therapeutic dose of 75 mg of PLAVIX per day.
A single oral dose or clopidogrel at 1500 or 2000 mg/kg was lethal to mice and to rats and at 3000 mg/kg to baboons. Symptoms of acute toxicity were vomiting (in baboons), prostration, difficult breathing, and gastrointestinal hemorrhage in all species.
Based on biological plausibility, platelet transfusion may be appropriate to reverse the pharmacological effects of PLAVIX if quick reversal is required.
The recommended dose of PLAVIX is 75 mg once daily with or without food.
No dosage adjustment is necessary for elderly patients or patients with renal disease. (See Clinical Pharmacology : Special Populations .)
PLAVIX (clopidogrel bisulfate) is available as a pink, round, biconvex, film-coated tablet debossed with "75" on one side and "1171" on the other. Tablets are provided as follows:
NDC 63653-1171-6 bottles of 30
NDC 63653-1171-1 bottles of 90
NDC 63653-1171-5 bottles of 500
NDC 63653-1171-3 blisters of 100
Store at 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F) [See USP Controlled Room Temperature]
Manufactured by:
Sanofi-Synthelabo Inc.
New York, NY 10016
Distributed by:
Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership
New York, NY 10016
PLAVIX® is a registered trademark of Sanofi-Synthelabo
Revised April 2000
1171 DIM-07 1081251AS
 |