 |
Each mL of Roxanol TM contains:
Morphine Sulfate .................................. 20 mg
Each mL of Roxanol TM -T contains:
Morphine Sulfate .................................. 20 mg
Solution is also tinted/flavored
Each 5 mL of Roxanol 100 TM contains:
Morphine Sulfate ................................ 100 mg
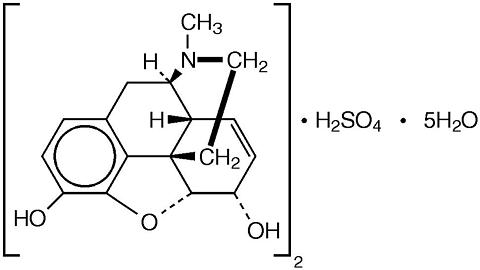
Chemically, Morphine Sulfate is, Morphinan-3,6-diol, 7,8-didehydro-4,5-epoxy-17-methyl-,(5(alpha),6(alpha))-, sulfate(2:1)(salt), pentahydrate, which can be represented by the following structural formula:
 |
Morphine Sulfate acts as a narcotic analgesic.
The major effects of morphine are on the central nervous system and the bowel. Opioids act as agonists, interacting with stereospecific and saturable binding sites or receptors in the brain and other tissues.
Morphine is about two-thirds absorbed from the gastrointestinal tract with the maximum analgesic effect occurring 60 minutes post administration.
Morphine is indicated for the relief of severe acute and severe chronic pain.
Hypersensitivity to morphine; respiratory insufficiency or depression; severe CNS depression; attack of bronchial asthma; heart failure secondary to chronic lung disease; cardiac arrhythmias; increased intracranial or cerebrospinal pressure; head injuries; brain tumor; acute alcoholism; delirium tremens; convulsive disorders; after biliary tract surgery; suspected surgical abdomen; surgical anastomosis; concomitantly with MAO inhibitors or within 14 days of such treatment.
Morphine can cause tolerance, psychological and physical dependence. Withdrawal will occur on abrupt discontinuation or administration of a narcotic antagonist.
Interaction with Other Central-Nervous-System Depressants --Morphine should be used with caution and in reduced dosage in patients who are concurrently receiving other narcotic analgesics, general anesthetics, phenothiazines, other tranquilizers, sedative-hypnotics, tricyclic antidepressants, and other CNS depressants (including alcohol). Respiratory depression, hypotension, and profound sedation or coma may result.
Head Injury and Increased Intracranial Pressure: The respiratory depressant effects of morphine and its capacity to elevate cerebrospinal-fluid pressure may be markedly exaggerated in the presence of increased intracranial pressure. Furthermore, narcotics produce side effects that may obscure the clinical course of patients with head injuries. In such patients, morphine must be used with caution and only if it is deemed essential.
Asthma and Other Respiratory Conditions: Morphine should be used with caution in patients having an acute asthmatic attack, in those with chronic obstructive pulmonary disease or cor pulmonale, and in individuals with a substantially decreased respiratory reserve, preexisting respiratory depression, hypoxia, or hypercapnia. In such patients, even usual therapeutic doses of narcotics may decrease respiratory drive while simultaneously increasing airway resistance to the point of apnea.
Hypotensive Effect: The administration of morphine may result in severe hypotension in an individual whose ability to maintain his blood pressure has already been compromised by a depleted blood volume or concurrent administration of such drugs as the phenothiazines or certain anesthetics.
Special-Risk Patients: Morphine should be given with caution and the initial dose should be reduced in certain patients, such as the elderly or debilitated and those with severe impairment of hepatic or renal function, hypothyroidism, Addison' disease, prostatic hypertrophy, or urethral stricture.
Acute Abdominal Conditions: The administration of morphine or other narcotics may obscure the diagnosis or clinical course in patients with acute abdominal conditions.
Use in Ambulatory Patients -- Morphine may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks, such as driving a car or operating machinery. The patient should be cautioned accordingly.
Morphine, like other narcotics, may produce orthostatic hypotension in ambulatory patients.
Patients should be cautioned about the combined effects of alcohol or other central nervous system depressants with morphine.
Generally, effects of morphine may be potentiated by alkalizing agents and antagonized by acidifying agents. Analgesic effect of morphine is potentiated by chlorpromazine and methocarbamol. CNS depressants such as anaesthetics, hypnotics, barbiturates, phenothiazines, chloral hydrate, glutethimide, sedatives, MAO inhibitors (including procarbazine hydrochloride), antihistamines, (beta)-blockers (propranolol), alcohol, furazolidone and other narcotics may enhance the depressant effects of morphine.
Morphine may increase anticoagulant activity of coumarin and other anticoagulants.
Long-term studies to determine the carcinogenic and mutagenic potential of morphine are not available.
Teratogenic Effects: Pregnancy Category C. Animal production studies have not been conducted with morphine. It is also not known whether morphine can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Morphine should be given to a pregnant woman only if clearly needed.
Morphine readily crosses the placental barrier and, if administered during labor, may lead to respiratory depression in the neonate.
Morphine has been detected in human milk. For this reason, caution should be exercised when morphine is administered to a nursing woman.
Safety and effectiveness in children have not been established.
THE MAJOR HAZARDS OF MORPHINE, AS OF OTHER NARCOTIC ANALGESICS, ARE RESPIRATORY DEPRESSION AND, TO A LESSER DEGREE, CIRCULATORY DEPRESSION, RESPIRATORY ARREST, SHOCK, AND CARDIAC ARREST HAVE OCCURRED.
The most frequently observed adverse reactions include lightheadedness, dizziness, sedation, nausea, vomiting, and sweating. These effects seem to be more prominent in ambulatory patients and in those who are not suffering severe pain. In such individuals, lower doses are available. Some adverse reactions may be alleviated in the ambulatory patient if he lies down.
Other adverse reactions include the following:
Central Nervous System: Euphoria, dysphoria, weakness, headache, insomnia, agitation, disorientation, and visual disturbances.
Gastrointestinal: Dry mouth, anorexia, constipation, and biliary tract spasm.
Cardiovascular: Flushing of the face, bradycardia, palpitation, faintness, and syncope.
Allergic: Pruritus, urticaria, other skin rashes, edema, and, rarely hemorrhagic urticaria.
Constipation: Ample intake of water or other liquids should be encouraged. Concomitant administration of a stool softener and a peristaltic stimulant with the narcotic analgesic can be an effective preventive measure for those patients in need of therapeutics. If elimination does not occur for two days, an enema should be administered to prevent impaction.
In the event diarrhea occurs, seepage around a fecal impaction is a possible cause to consider before antidiarrheal measures are employed.
Nausea and Vomiting: Phenothiazines and antihistamines can be effective treatments for nausea of the medullary and vestibular sources respectively. However, these drugs may potentiate the side effects of the narcotics or the antinauseant.
Drowsiness (sedation): Once pain control is achieved, undesirable sedation can be minimized by titrating the dosage to a level that just maintains a tolerable pain or pain free state.
Morphine Sulfate, a narcotic, is a Schedule II controlled substance under the Federal Controlled Substance Act. As with other narcotics, some patients may develop a physical and psychological dependence on morphine. They may increase dosage without consulting a physician and subsequently may develop a physical dependence on the drug. In such cases, abrupt discontinuance may precipitate typical withdrawal symptoms, including convulsions. Therefore the drug should be withdrawn gradually from any patient known to be taking excessive dosages over a long period of time.
In treating the terminally ill patient the benefit of pain relief may outweigh the possibility of drug dependence. The chance of drug dependence is substantially reduced when the patient is placed on scheduled narcotic programs instead of a "pain to relief-of-pain" cycle typical of a PRN regimen.
Signs and Symptoms: Serious overdose with morphine is characterized by respiratory depression (a decrease in respiratory rate and/or tidal volume, Cheyne-Stokes respiration, cyanosis), extreme somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold or clammy skin, and sometimes bradycardia and hypotension. In severe overdosage, apnea, circulatory collapse, cardiac arrest and death may occur.
Treatment: Primary attention should be given to the re-establishment of adequate respiratory exchange through provision of a patent airway and the institution of assisted or controlled ventilation. The narcotic antagonist naloxone is a specific antidote against respiratory depression which may result from overdosage or unusual sensitivity to narcotics, including morphine. Therefore, an appropriate dose of naloxone (usual initial adult dose: 0.4 mg) should be administered, preferably by the intravenous route and simultaneously with efforts at respiratory resuscitation. Since the duration of action of morphine may exceed that of the antagonist, the patient should be kept under continued surveillance and repeated doses of the antagonist should be administered as needed to maintain adequate respiration.
An antagonist should not be administered in the absence of clinically significant respiratory or cardiovascular depression.
Oxygen, intravenous fluids, vasopressors and other supportive measures should be employed as indicated.
Gastric emptying may be useful in removing unabsorbed drug.
Usual Adult Oral Dose: 10 to 30 mg every 4 hours or as directed by physician. Dosage is a patient dependent variable, therefore increased dosage may be required to achieve adequate analgesia.
For control of severe, chronic pain in patients with certain terminal disease, this drug should be administered on a regularly scheduled basis, every 4 hours, at the lowest dosage level that will achieve adequate analgesia.
Note: Medication may suppress respiration in the elderly, the very ill, and those patients with respiratory problems, therefore lower doses may be required.
Morphine Dosage Reduction: During the first two to three days of effective pain relief, the patient may sleep for many hours. This can be misinterpreted as the effect of excessive analgesic dosing rather than the first sign of relief in a pain exhausted patient. The dose, therefore, should be maintained for at least three days before reduction, if respiratory activity and other vital signs are adequate.
Following successful relief of severe pain, periodic attempts to reduce the narcotic dose should be made. Smaller doses or complete discontinuation of the narcotic analgesic may become feasible due to a physiologic change or the improved mental state of the patient.
Morphine Sulfate (Immediate Release)
20 mg per mL
NDC 0054-3751-44: Bottles of 30 mL with calibrated dropper.
NDC 0054-3751-50: Bottles of 120 mL with calibrated dropper.
Roxanol TM -T
Morphine Sulfate (Immediate Release)
20 mg per mL (tinted/flavored)
NDC 0054-3774-44: Bottles of 30 mL with calibrated dropper.
NDC 0054-3774-50: Bottles of 120 mL with calibrated dropper.
Morphine Sulfate (Immediate Release)
100 mg per 5 mL
NDC 0054-3751-58: Bottles of 240 mL with calibrated patient spoon.
DEA Order Form Required
4073001 Revised February 1999
029 ©RLI, 1999
Roxane Laboratories, Inc.
Columbus, OH 43216
 |
 |
 |