 |
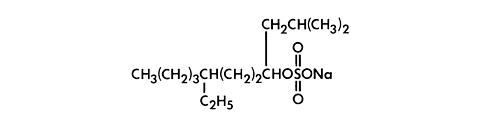
Sodium tetradecyl sulfate is an anionic surfactant which occurs as a white, waxy solid. The structural formula is as follows:
 |
C 14 H 29 NaSO 4
7-Ethyl-2-methyl-4-hendecanol sulfate sodium salt
M.W. 316.44
Sotradecol® (Sodium Tetradecyl Sulfate Injection) is a sterile nonpyrogenic solution for intravenous use as a sclerosing agent. Each mL contains sodium tetradecyl sulfate 10 mg or 30 mg, benzyl alcohol 0.02 mL and dibasic sodium phosphate, anhydrous 0.72 mg in Water for Injection. pH 7.9; monobasic sodium phosphate and/or sodium hydroxide added, if needed, for pH adjustment.
Sotradecol® (Sodium Tetradecyl Sulfate Injection) is a mild sclerosing agent. Intravenous injection causes intima inflammation and thrombus formation. This usually occludes the injected vein. Subsequent formation of fibrous tissue results in partial or complete vein obliteration.
Indicated in the treatment of small uncomplicated varicose veins of the lower extremities that show simple dilation with competent valves. The benefit-to-risk ratio should be considered in selected patients who are great surgical risks.
Contraindicated in previous hypersensitivity reactions to the drug; in acute superficial thrombophlebitis; significant valvular or deep vein incompetence; huge superficial veins with wide open communications to deeper veins; phlebitis migrans; acute cellulitis; allergic conditions; acute infections; varicosities caused by abdominal and pelvic tumors unless the tumor has been removed; bedridden patients; such uncontrolled systemic diseases as diabetes, toxic hyperthyroidism, tuberculosis, asthma, neoplasm, sepsis, blood dyscrasias and acute respiratory or skin diseases.
Since severe adverse local effects, including tissue necrosis, may occur following extravasation, Sotradecol® (Sodium Tetradecyl Sulfate Injection), should be administered only by a physician familiar with proper injection technique. Extreme care in needle placement and using the minimal effective volume at each injection site are, therefore, important.
Allergic reactions, including anaphylaxis, have been reported that led to death. Therefore, as a precaution against anaphylactic shock, it is recommended that 0.5 mL of Sotradecol® be injected into a varicosity, followed by observation of the patient for several hours before administration of a second or larger dose. The possibility of an anaphylactic reaction should be kept in mind, and the physician should be prepared to treat it appropriately. In extreme emergencies, 0.25 mL of 1:1000 Epinephrine Injection (0.25 mg) intravenously should be used and side reactions controlled with antihistamines.
The drug should only be administered by physicians who are familiar with an acceptable injection technique. Because of the danger of thrombosis extension into the deep venous system, thorough preinjection evaluation for valvular competency should be carried out and slow injections with a small amount (not over 2 mL) of the preparation should be injected into the varicosity. In particular, deep venous patency must be determined by angiography and/or the Perthes test before sclerotherapy is undertaken. Venous sclerotherapy should not be undertaken if tests, such as the Trendelenberg and Perthes, and angiography show significant valvular or deep venous incompetence. The physician should bear in mind that injection necrosis is likely to result from extravascular injection of sclerosing agents.
Extreme caution must be exercised in the presence of underlying arterial disease such as marked peripheral arteriosclerosis or thromboangiitis obliterans (Buerger's Disease).
Embolism may occur as long as four weeks after injection of sodium tetradecyl sulfate. The incidence of recurrence is low if the patient wears elastic stockings.
No well-controlled studies have been performed on patients taking antiovulatory agents. The physician must use judgment and evaluate any patient taking antiovulatory drugs prior to initiating treatment with Sotradecol®. (See ADVERSE REACTIONS .)
Heparin should not be included in the same syringe as Sotradecol®, since the two are incompatible.
When tested in the L5178YTK + / - mouse lymphoma assay, sodium tetradecyl sulfate did not induce a dose-related increase in the frequency of thymidine kinase-deficient mutants and, therefore, was judged to be nonmutagenic in this system. However, no long-term animal carcinogenicity studies with sodium tetradecyl sulfate have been performed.
Teratogenic Effects--Pregnancy Category C. Animal reproduction studies have not been conducted with Sotradecol®. It is also not known whether Sotradecol® can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Sotradecol® should be given to a pregnant woman only if clearly needed.
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Sotradecol® is administered to a nursing woman.
Safety and effectiveness in pediatric patients have not been established.
Local reactions consisting of pain, urticaria or ulceration may occur at the site of injection. A permanent discoloration, usually small and hardly noticeable but which may be objectionable from a cosmetic viewpoint, may remain along the path of the sclerosed vein segment. Sloughing and necrosis of tissue may occur following extravasation of the drug.
Allergic reactions such as hives, asthma, hayfever and anaphylactic shock have been reported. Mild systemic reactions that have been reported include headache, nausea and vomiting. (See .)
Four deaths have been reported with the use of Sotradecol®. One death has been reported in a patient who received Sotradecol® and who had been receiving an antiovulatory agent. Another death (fatal pulmonary embolism) has been reported in a 36-year-old female treated with sodium tetradecyl acetate and who was not taking oral contraceptives. Two cases of anaphylactic shock leading to death have been reported in patients who received Sotradecol®. One of the patients reported a medical history of asthma, a contraindication to the administration of Sotradecol®.
For intravenous use only. Do not use if precipitated or discolored. The strength of solution required depends on the size and degree of varicosity. In general, the 1% solution will be found most useful with the 3% solution preferred for larger varicosities. The dosage should be kept small, using 0.5 to 2 mL (preferably 1 mL maximum) for each injection, and the maximum single treatment should not exceed 10 mL.
Sotradecol® (Sodium Tetradecyl Sulfate Injection)
1%--2 mL DOSETTE® ampuls packaged in 5s (NDC 0641-1514-34)
3%--2 mL DOSETTE® ampuls packaged in 5s (NDC 0641-1516-34)
Store at controlled room temperature 15°-30°C (59°-86°F).
The intravenous LD 50 of sodium tetradecyl sulfate in mice was reported to be 90 ± 5 mg/kg.
In the rat, the acute intravenous LD 50 of sodium tetradecyl sulfate was estimated to be between 72 mg/kg and 108 mg/kg.
Purified sodium tetradecyl sulfate was found to have an LD 50 of 2 g/kg when administered orally by stomach tube as a 25% aqueous solution to rats. In rats given 0.15 g/kg in drinking water for 30 days, no appreciable toxicity was seen, although some growth inhibition was discernible.
* * * *
ELKINS-SINN, INC., Cherry Hill, NJ 08003-4099
A division of A.H. Robins Company
J-1514H Revised November 1996