 |
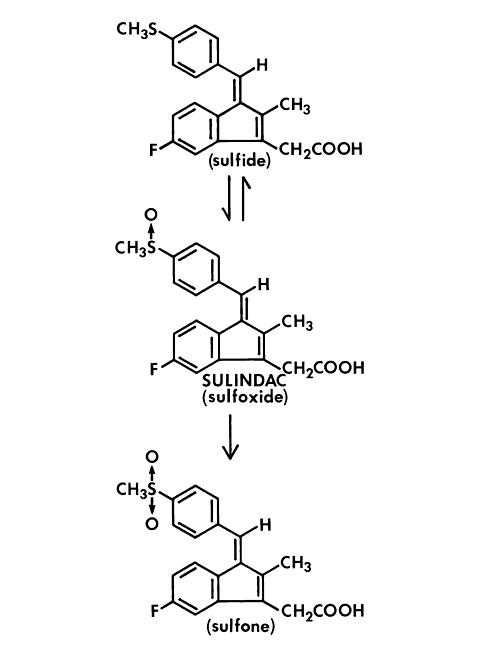
Sulindac is a non-steroidal, anti-inflammatory indene derivative designated chemically as (Z)- 5-fluoro-2-methyl - 1 - [ [ p - (methylsulfinyl) phenyl]methylene]-1 H -indene-3-acetic acid. It is not a salicylate, pyrazolone or propionic acid derivative. Its empirical formula is C 20 H 17 FO 3 S, with a molecular weight of 356.42. Sulindac, a yellow crystalline compound, is a weak organic acid practically insoluble in water below pH 4.5, but very soluble as the sodium salt or in buffers of pH 6 or higher.
CLINORIL* (Sulindac) is available in 150 and 200 mg tablets for oral administration. Each tablet contains the following inactive ingredients: cellulose, magnesium stearate, starch.
Following absorption, sulindac undergoes two major biotransformations--reversible reduction to the sulfide metabolite, and irreversible oxidation to the sulfone metabolite. Available evidence indicates that the biological activity resides with the sulfide metabolite.
The structural formulas of sulindac and its metabolites are:
 |
*Registered trademark of MERCK & CO., INC.
CLINORIL is a non-steroidal anti-inflammatory drug, also possessing analgesic and antipyretic activities. Its mode of action, like that of other non-steroidal anti-inflammatory agents, is not known; however, its therapeutic action is not due to pituitary-adrenal stimulation. Inhibition of prostaglandin synthesis by the sulfide metabolite may be involved in the anti-inflammatory action of CLINORIL.
Sulindac is approximately 90% absorbed in man after oral administration. The peak plasma concentrations of the biologically active sulfide metabolite are achieved in about two hours when sulindac is administered in the fasting state, and in about three to four hours when sulindac is administered with food. The mean half-life of sulindac is 7.8 hours while the mean half-life of the sulfide metabolite is 16.4 hours. Sustained plasma levels of the sulfide metabolite are consistent with a prolonged anti-inflammatory action which is the rationale for a twice per day dosage schedule.
Sulindac and its sulfone metabolite undergo extensive enterohepatic circulation relative to the sulfide metabolite in animals. Studies in man have also demonstrated that recirculation of the parent drug, sulindac, and its sulfone metabolite, is more extensive than that of the active sulfide metabolite. The active sulfide metabolite accounts for less than six percent of the total intestinal exposure to sulindac and its metabolites.
The primary route of excretion in man is via the urine as both sulindac and its sulfone metabolite (free and glucuronide conjugates). Approximately 50% of the administered dose is excreted in the urine, with the conjugated sulfone metabolite accounting for the major portion. Less than 1% of the administered dose of sulindac appears in the urine as the sulfide metabolite. Approximately 25% is found in the feces, primarily as the sulfone and sulfide metabolites.
The bioavailability of sulindac, as assessed by urinary excretion, was not changed by concomitant administration of an antacid containing magnesium hydroxide 200 mg and aluminum hydroxide 225 mg per 5 mL.
Because CLINORIL is excreted in the urine primarily as biologically inactive forms, it may possibly affect renal function to a lesser extent than other non-steroidal anti-inflammatory drugs, however, renal adverse experiences have been reported with CLINORIL (see ADVERSE REACTIONS ). In a study of patients with chronic glomerular disease treated with therapeutic doses of CLINORIL, no effect was demonstrated on renal blood flow, glomerular filtration rate, or urinary excretion of prostaglandin E 2 and the primary metabolite of prostacyclin, 6-keto-PGF 1 (alpha). However, in other studies in healthy volunteers and patients with liver disease, CLINORIL was found to blunt the renal responses to intravenous furosemide, i.e., the diuresis, natriuresis, increments in plasma renin activity and urinary excretion of prostaglandins. These observations may represent a differentiation of the effects of CLINORIL on renal functions based on differences in pathogenesis of the renal prostaglandin dependence associated with differing dose-response relationships of different NSAIDs to the various renal functions influenced by prostaglandins. These observations need further clarification and in the interim, sulindac should be used with caution in patients whose renal function may be impaired (see PRECAUTIONS ).
In healthy men, the average fecal blood loss, measured over a two-week period during administration of 400 mg per day of CLINORIL, was similar to that for placebo, and was statistically significantly less than that resulting from 4800 mg per day of aspirin.
In controlled clinical studies CLINORIL was evaluated in the following five conditions:
CLINORIL is indicated for acute or long-term use in the relief of signs and symptoms of the following:
**The safety and effectiveness of CLINORIL have not been established in rheumatoid arthritis patients who are designated in the American Rheumatism Association classification as Functional Class IV (incapacitated, largely or wholly bedridden, or confined to wheelchair; little or no self-care).
CLINORIL should not be used in:
Patients who are hypersensitive to this product.
Patients in whom acute asthmatic attacks, urticaria, or rhinitis are precipitated by aspirin or other non-steroidal anti-inflammatory agents.
Gastrointestinal Effects
Peptic ulceration and gastrointestinal bleeding have been reported in patients receiving CLINORIL. Fatalities have occurred. Gastrointestinal bleeding is associated with higher morbidity and mortality in patients acutely ill with other conditions, the elderly and patients with hemorrhagic disorders. In patients with active gastrointestinal bleeding or an active peptic ulcer, an appropriate ulcer regimen should be instituted, and the physician must weigh the benefits of therapy with CLINORIL against possible hazards, and carefully monitor the patient' progress. When CLINORIL is given to patients with a history of either upper or lower gastrointestinal tract disease, it should be given under close supervision and only after consulting the ADVERSE REACTIONS section.
Risk of GI Ulcerations, Bleeding and Perforation with NSAID Therapy
Serious gastrointestinal toxicity such as bleeding, ulceration, and perforation can occur at any time, with or without warning symptoms, in patients treated chronically with NSAID therapy. Although minor upper gastrointestinal problems, such as dyspepsia, are common, usually developing early in therapy, physicians should remain alert for ulceration and bleeding in patients treated chronically with NSAIDs even in the absence of previous GI tract symptoms. In patients observed in clinical trials of several months to two years duration, symptomatic upper GI ulcers, gross bleeding or perforation appear to occur in approximately 1% of patients treated for 3-6 months, and in about 2-4% of patients treated for one year. Physicians should inform patients about the signs and/or symptoms of serious GI toxicity and what steps to take if they occur.
Studies to date have not identified any subset of patients not at risk of developing peptic ulceration and bleeding. Except for a prior history of serious GI events and other risk factors known to be associated with peptic ulcer disease, such as alcoholism, smoking, etc., no risk factors (e.g., age, sex) have been associated with increased risk. Elderly or debilitated patients seem to tolerate ulceration or bleeding less well than other individuals and most spontaneous reports of fatal GI events are in this population. Studies to date are inconclusive concerning the relative risk of various NSAIDs in causing such reactions. High doses of any NSAID probably carry a greater risk of these reactions, although controlled clinical trials showing this do not exist in most cases. In considering the use of relatively large doses (within the recommended dosage range), sufficient benefit should be anticipated to offset the potential increased risk of GI toxicity.
Hypersensitivity
Rarely, fever and other evidence of hypersensitivity (see ADVERSE REACTIONS ) including abnormalities in one or more liver function tests and severe skin reactions have occurred during therapy with CLINORIL. Fatalities have occurred in these patients. Hepatitis, jaundice, or both, with or without fever, may occur usually within the first one to three months of therapy. Determinations of liver function should be considered whenever a patient on therapy with CLINORIL develops unexplained fever, rash or other dermatologic reactions or constitutional symptoms. If unexplained fever or other evidence of hypersensitivity occurs, therapy with CLINORIL should be discontinued. The elevated temperature and abnormalities in liver function caused by CLINORIL characteristically have reverted to normal after discontinuation of therapy. Administration of CLINORIL should not be reinstituted in such patients.
Hepatic Effects
In addition to hypersensitivity reactions involving the liver, in some patients the findings are consistent with those of cholestatic hepatitis. As with other non-steroidal anti-inflammatory drugs, borderline elevations of one or more liver tests without any other signs and symptoms may occur in up to 15% of patients. These abnormalities may progress, may remain essentially unchanged, or may be transient with continued therapy. The SGPT (ALT) test is probably the most sensitive indicator of liver dysfunction. Meaningful (3 times the upper limit of normal) elevations of SGPT or SGOT (AST) occurred in controlled clinical trials in less than 1% of patients. A patient with symptoms and/or signs suggesting liver dysfunction, or in whom an abnormal liver test has occurred, should be evaluated for evidence of the development of more severe hepatic reaction while on therapy with CLINORIL. Although such reactions as described above are rare, if abnormal liver tests persist or worsen, if clinical signs and symptoms consistent with liver disease develop, or if systemic manifestations occur (e.g. eosinophilia, rash, etc.), CLINORIL should be discontinued.
In clinical trials with CLINORIL, the use of doses of 600 mg/ day has been associated with an increased incidence of mild liver test abnormalities (see DOSAGE AND ADMINISTRATION for maximum dosage recommendation).
General
Non-steroidal anti-inflammatory drugs, including CLINORIL, may mask the usual signs and symptoms of infection. Therefore, the physician must be continually on the alert for this and should use the drug with extra care in the presence of existing infection.
Although CLINORIL has less effect on platelet function and bleeding time than aspirin, it is an inhibitor of platelet function; therefore, patients who may be adversely affected should be carefully observed when CLINORIL is administered.
Pancreatitis has been reported in patients receiving CLINORIL (see ADVERSE REACTIONS ). Should pancreatitis be suspected, the drug should be discontinued and not restarted, supportive medical therapy instituted, and the patient monitored closely with appropriate laboratory studies (e.g., serum and urine amylase, amylase/creatinine clearance ratio, electrolytes, serum calcium, glucose, lipase, etc.). A search for other causes of pancreatitis as well as those conditions which mimic pancreatitis should be conducted.
Because of reports of adverse eye findings with non-steroidal anti-inflammatory agents, it is recommended that patients who develop eye complaints during treatment with CLINORIL have ophthalmologic studies.
In patients with poor liver function, delayed, elevated and prolonged circulating levels of the sulfide and sulfone metabolites may occur. Such patients should be monitored closely; a reduction of daily dosage may be required.
Edema has been observed in some patients taking CLINORIL. Therefore, as with other non-steroidal anti-inflammatory drugs, CLINORIL should be used with caution in patients with compromised cardiac function, hypertension, or other conditions predisposing to fluid retention.
CLINORIL may allow a reduction in dosage or the elimination of chronic corticosteroid therapy in some patients with rheumatoid arthritis. However, it is generally necessary to reduce corticosteroids gradually over several months in order to avoid an exacerbation of disease or signs and symptoms of adrenal insufficiency. Abrupt withdrawal of chronic corticosteroid treatment is generally not recommended even when patients have had a serious complication of chronic corticosteroid therapy.
Renal Effects
As with other non-steroidal anti-inflammatory drugs, long-term administration of sulindac to animals has resulted in renal papillary necrosis and other abnormal renal pathology. In humans, there have been reports of acute interstitial nephritis with hematuria, proteinuria, and occasionally nephrotic syndrome.
A second form of renal toxicity has been seen in patients with prerenal and renal conditions leading to a reduction in renal blood flow or blood volume, where the renal prostaglandins have a supportive role in the maintenance of renal perfusion. In these patients, administration of an NSAID may cause a dose dependent reduction in prostaglandin formation and may precipitate overt renal decompensation. CLINORIL may affect renal function less than other NSAIDs in patients with chronic glomerular renal disease (see ). Until these observations are better understood and clarified, however, and because renal adverse experiences have been reported with CLINORIL (see ADVERSE REACTIONS ), caution should be exercised when administering the drug to patients with conditions associated with increased risk of the effects of non-steroidal anti-inflammatory drugs on renal function, such as those with renal or hepatic dysfunction, diabetes mellitus, advanced age, extracellular volume depletion from any cause, congestive heart failure, septicemia, pyelonephritis, or concomitant use of any nephrotoxic drug. Discontinuation of NSAID therapy is typically followed by recovery to the pretreatment state.
Since CLINORIL is eliminated primarily by the kidneys, patients with significantly impaired renal function should be closely monitored; a lower daily dosage should be anticipated to avoid excessive drug accumulation.
Sulindac metabolites have been reported rarely as the major or a minor component in renal stones in association with other calculus components. CLINORIL should be used with caution in patients with a history of renal lithiasis, and they should be kept well hydrated while receiving CLINORIL.
Information for Patients
CLINORIL, like other drugs of its class, is not free of side effects. The side effects of these drugs can cause discomfort and, rarely, there are more serious side effects such as gastrointestinal bleeding, which may result in hospitalization and even fatal outcomes.
NSAIDs (Non-steroidal Anti-inflammatory Drugs) are often essential agents in the management of arthritis, but they also may be commonly employed for conditions which are less serious.
Physicians may wish to discuss with their patients the potential risks (see , PRECAUTIONS and ADVERSE REACTIONS ) and likely benefits of NSAID treatment, particularly when the drugs are used for less serious conditions where treatment without NSAIDs may represent an acceptable alternative to both the patient and physician.
Laboratory Tests
Because serious GI tract ulceration and bleeding can occur without warning symptoms, physicians should follow chronically treated patients for the signs and symptoms of ulceration and bleeding and should inform them of the importance of this follow-up (see , Risk of GI Ulcerations, Bleeding and Perforation with NSAID Therapy ).
Use in Pregnancy
CLINORIL is not recommended for use in pregnant women, since safety for use has not been established. The known effects of drugs of this class on the human fetus during the third trimester of pregnancy include: constriction of the ductus arteriosus prenatally, tricuspid incompetence, and pulmonary hypertension; non-closure of the ductus arteriosus postnatally which may be resistant to medical management; myocardial degenerative changes, platelet dysfunction with resultant bleeding, intracranial bleeding, renal dysfunction or failure, renal injury/dysgenesis which may result in prolonged or permanent renal failure, oligohydramnios, gastrointestinal bleeding or perforation, and increased risk of necrotizing enterocolitis.
In reproduction studies in the rat, a decrease in average fetal weight and an increase in numbers of dead pups were observed on the first day of the postpartum period at dosage levels of 20 and 40 mg/kg/day (2 1 / 2 and 5 times the usual maximum daily dose in humans), although there was no adverse effect on the survival and growth during the remainder of the postpartum period. CLINORIL prolongs the duration of gestation in rats, as do other compounds of this class which also may cause dystocia and delayed parturition in pregnant animals. Visceral and skeletal malformations observed in low incidence among rabbits in some teratology studies did not occur at the same dosage levels in repeat studies, nor at a higher dosage level in the same species.
Nursing Mothers
Nursing should not be undertaken while a patient is on CLINORIL. It is not known whether sulindac is secreted in human milk; however, it is secreted in the milk of lactating rats.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatric Use
As with any NSAID, caution should be exercised in treating the elderly (65 years and older) since advancing age appears to increase the possibility of adverse reactions. Elderly patients seem to tolerate ulceration or bleeding less well than other individuals and many spontaneous reports of fatal GI events are in this population (see , Gastrointestinal Effects and Risk of GI Ulcerations, Bleeding and Perforation with NSAID Therapy ).
This drug is known to be substantially excreted by the kidney and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection and it may be useful to monitor renal function (see PRECAUTIONS , Renal Effects ).
DMSO should not be used with sulindac. Concomitant administration has been reported to reduce the plasma levels of the active sulfide metabolite and potentially reduce efficacy. In addition, this combination has been reported to cause peripheral neuropathy.
Although sulindac and its sulfide metabolite are highly bound to protein, studies, in which CLINORIL was given at a dose of 400 mg daily, have shown no clinically significant interaction with oral anticoagulants or oral hypoglycemic agents. However, patients should be monitored carefully until it is certain that no change in their anticoagulant or hypoglycemic dosage is required. Special attention should be paid to patients taking higher doses than those recommended and to patients with renal impairment or other metabolic defects that might increase sulindac blood levels.
The concomitant administration of aspirin with sulindac significantly depressed the plasma levels of the active sulfide metabolite. A double-blind study compared the safety and efficacy of CLINORIL 300 or 400 mg daily given alone or with aspirin 2.4 g/day for the treatment of osteoarthritis. The addition of aspirin did not alter the types of clinical or laboratory adverse experiences for CLINORIL; however, the combination showed an increase in the incidence of gastrointestinal adverse experiences. Since the addition of aspirin did not have a favorable effect on the therapeutic response to CLINORIL, the combination is not recommended.
The concomitant use of CLINORIL with other NSAIDs is not recommended due to the increased possibility of gastrointestinal toxicity, with little or no increase in efficacy.
Caution should be used if CLINORIL is administered concomitantly with methotrexate. Nonsteroidal anti-inflammatory drugs have been reported to decrease the tubular secretion of methotrexate and to potentiate its toxicity.
Administration of non-steroidal anti-inflammatory drugs concomitantly with cyclosporine has been associated with an increase in cyclosporine-induced toxicity, possibly due to decreased synthesis of renal prostacyclin. NSAIDs should be used with caution in patients taking cyclosporine, and renal function should be carefully monitored.
The concomitant administration of CLINORIL and diflunisal in normal volunteers resulted in lowering of the plasma levels of the active sulindac sulfide metabolite by approximately one-third.
Probenecid given concomitantly with sulindac had only a slight effect on plasma sulfide levels, while plasma levels of sulindac and sulfone were increased. Sulindac was shown to produce a modest reduction in the uricosuric action of probenecid, which probably is not significant under most circumstances.
Neither propoxyphene hydrochloride nor acetaminophen had any effect on the plasma levels of sulindac or its sulfide metabolite.
The following adverse reactions were reported in clinical trials or have been reported since the drug was marketed. The probability exists of a causal relationship between CLINORIL and these adverse reactions. The adverse reactions which have been observed in clinical trials encompass observations in 1,865 patients, including 232 observed for at least 48 weeks.
Incidence Greater Than 1%
The most frequent types of adverse reactions occurring with CLINORIL are gastrointestinal; these include gastrointestinal pain (10%), dyspepsia***, nausea*** with or without vomiting, diarrhea***, constipation***, flatulence, anorexia and gastrointestinal cramps.
Dermatologic
Rash***, pruritus.
Dizziness***, headache***, nervousness.
Special Senses
Tinnitus.
Miscellaneous
Edema (see PRECAUTIONS ).
***Incidence between 3% and 9%. Those reactions occurring in 1% to 3% of patients are not marked with an asterisk.
Incidence Less Than 1 in 100
Gastritis, gastroenteritis or colitis. Peptic ulcer and gastrointestinal bleeding have been reported. GI perforation and intestinal strictures (diaphragms) have been reported rarely.
Liver function abnormalities; jaundice, sometimes with fever; cholestasis; hepatitis; hepatic failure.
There have been rare reports of sulindac metabolites in common bile duct "sludge" and in biliary calculi in patients with symptoms of cholecystitis who underwent a cholecystectomy.
Pancreatitis (see PRECAUTIONS ).
Ageusia; glossitis.
Dermatologic
Stomatitis, sore or dry mucous membranes, alopecia, photosensitivity.
Erythema multiforme, toxic epidermal necrolysis, Stevens-Johnson syndrome, and exfoliative dermatitis have been reported.
Congestive heart failure, especially in patients with marginal cardiac function; palpitation; hypertension.
Hematologic
Thrombocytopenia; ecchymosis; purpura; leukopenia; agranulocytosis; neutropenia; bone marrow depression, including aplastic anemia; hemolytic anemia; increased prothrombin time in patients on oral anticoagulants (see PRECAUTIONS ).
Urine discoloration; dysuria; vaginal bleeding; hematuria; proteinuria; crystalluria; renal impairment, including renal failure; interstitial nephritis; nephrotic syndrome.
Renal calculi containing sulindac metabolites have been observed rarely.
Hyperkalemia.
Muscle weakness.
Depression; psychic disturbances including acute psychosis.
Vertigo; insomnia; somnolence; paresthesia; convulsions; syncope; aseptic meningitis.
Special Senses
Blurred vision; visual disturbances; decreased hearing; metallic or bitter taste.
Respiratory
Epistaxis.
Hypersensitivity Reactions
Anaphylaxis; angioneurotic edema; bronchial spasm; dyspnea.
Hypersensitivity vasculitis.
A potentially fatal apparent hypersensitivity syndrome has been reported. This syndrome may include constitutional symptoms (fever, chills, diaphoresis, flushing), cutaneous findings (rash or other dermatologic reactions--see above), conjunctivitis, involvement of major organs (changes in liver function including hepatic failure, jaundice, pancreatitis, pneumonitis with or without pleural effusion, leukopenia, leukocytosis, eosinophilia, disseminated intravascular coagulation, anemia, renal impairment, including renal failure), and other less specific findings (adenitis, arthralgia, arthritis, myalgia, fatigue, malaise, hypotension, chest pain, tachycardia).
Causal Relationship Unknown
A rare occurrence of fulminant necrotizing fasciitis, particularly in association with Group A (beta)-hemolytic streptococcus, has been described in persons treated with non-steroidal anti-inflammatory agents, sometimes with fatal outcome (see also PRECAUTIONS , General ).
Other reactions have been reported in clinical trials or since the drug was marketed, but occurred under circumstances where a causal relationship could not be established. However, in these rarely reported events, that possibility cannot be excluded. Therefore, these observations are listed to serve as alerting information to physicians.
Arrhythmia.
Hyperglycemia.
Neuritis.
Special Senses
Disturbances of the retina and its vasculature.
Miscellaneous
Gynecomastia.
Cases of overdosage have been reported and rarely, deaths have occurred. The following signs and symptoms may be observed following overdosage: stupor, coma, diminished urine output and hypotension.
In the event of overdosage, the stomach should be emptied by inducing vomiting or by gastric lavage, and the patient carefully observed and given symptomatic and supportive treatment.
Animal studies show that absorption is decreased by the prompt administration of activated charcoal and excretion is enhanced by alkalinization of the urine.
CLINORIL should be administered orally twice a day with food. The maximum dosage is 400 mg per day. Dosages above 400 mg per day are not recommended.
In osteoarthritis, rheumatoid arthritis, and ankylosing spondylitis, the recommended starting dosage is 150 mg twice a day. The dosage may be lowered or raised depending on the response.
A prompt response (within one week) can be expected in about one-half of patients with osteoarthritis, ankylosing spondylitis, and rheumatoid arthritis. Others may require longer to respond.
In acute painful shoulder (acute subacromial bursitis/supraspinatus tendinitis) and acute gouty arthritis, the recommended dosage is 200 mg twice a day. After a satisfactory response has been achieved, the dosage may be reduced according to the response. In acute painful shoulder, therapy for 7-14 days is usually adequate. In acute gouty arthritis, therapy for 7 days is usually adequate.
No. 3360--Tablets CLINORIL 150 mg are bright yellow, hexagon-shaped, compressed tablets, coded MSD 941 on one side and CLINORIL on the other. They are supplied as follows:
NDC 0006-0941-68 in bottles of 100
(6505-01-071-5559, 150 mg 100's).
No. 3353--Tablets CLINORIL 200 mg are bright yellow, hexagon-shaped, scored, compressed tablets, coded MSD 942 on one side and CLINORIL on the other. They are supplied as follows:
NDC 0006-0942-68 in bottles of 100
(6505-01-072-3426, 200 mg 100's).
7858637 Issued July 1998
COPYRIGHT © MERCK & CO., INC., 1988
All rights reserved
 |