 |
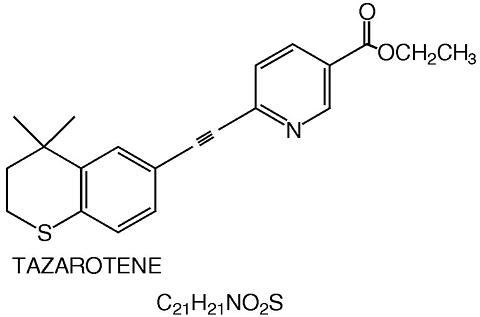
TAZORAC® is a translucent, aqueous gel and contains the compound tazarotene, a member of the acetylenic class of retinoids. It is for topical dermatologic use only. The active ingredient is represented by the following structural formula:
 |
Chemical Name : ethyl 6-[2-(4,4-dimethylthiochroman-6-yl)-ethynyl] nicotinate
Contains:
Active
: Tazarotene ..................... 0.05% or 0.1% (w/w)
Preservative
: Benzyl alcohol ..................... 1.0% (w/w)
Inactives: Ascorbic acid, butylated hydroxyanisole, butylated hydroxytoluene, carbomer 934P, edetate disodium, hexylene glycol, purified water, poloxamer 407, polyethylene glycol 400, polysorbate 40, and tromethamine.
Tazarotene is a retinoid prodrug which is converted to its active form, the cognate carboxylic acid of tazarotene (AGN 190299), by rapid deesterification in most biological systems. AGN 190299 binds to all three members of the retinoic acid receptor (RAR) family: RAR(alpha), RAR(beta), and RAR(gamma), but shows relative selectivity for RAR(beta), and RAR(gamma) and may modify gene expression. The clinical significance of these findings is unknown.
Psoriasis : The mechanism of tazarotene action in psoriasis is not defined. Topical tazarotene blocks induction of mouse epidermal ornithine decarboxylase (ODC) activity, which is associated with cell proliferation and hyperplasia. In cell culture and in vitro models of skin, tazarotene suppresses expression of MRP8, a marker of inflammation present in the epidermis of psoriasis subjects at high levels. In human keratinocyte cultures, it inhibits cornified envelope formation, whose build-up is an element of the psoriatic scale. The clinical significance of these findings is unknown.
Acne : The mechanism of tazarotene action in acne is not defined. Tazarotene inhibited corneocyte accumulation in rhino mouse skin and cross-linked envelope formation in cultured human keratinocytes. The clinical significance of these findings is unknown.
Following topical application, tazarotene undergoes esterase hydrolysis to form its active metabolite, AGN 190299. Little parent compound could be detected in the plasma. AGN 190299 was highly bound to plasma proteins (>99%). Tazarotene and AGN 190299 were metabolized to sulfoxides, sulfones and other polar metabolites which were eliminated through urinary and fecal pathways. The half-life of AGN 190299 following topical application of tazarotene was similar in normal and psoriatic subjects, approximately 18 hours.
The human in vivo studies described below were conducted with tazarotene gel applied topically at approximately 2 mg/cm 2 and left on the skin for 10 to 12 hours. Both the peak plasma concentration (Cmax) and area under the plasma concentration time curve (AUC) refer to the active metabolite only.
Two single, topical dose studies were conducted using 14 C-tazarotene gel. Systemic absorption, as determined from radioactivity in the excreta, was less than 1% of the applied dose (without occlusion) in six psoriatic patients and approximately 5% of the applied dose (under occlusion) in six healthy subjects. One non-radiolabeled single-dose study comparing the 0.05% gel to the 0.1% gel in healthy subjects indicated that the Cmax and AUC were 40% higher for the 0.1% gel.
After 7 days of topical dosing with measured doses of tazarotene 0.1% gel on 20% of the total body surface without occlusion in 24 healthy subjects, the Cmax was 0.72 ± 0.58 ng/mL (mean ± SD) occurring 9 hours after the last dose, and the AUC 0-24hr was 10.1 ± 7.2 ng·hr/mL. Systemic absorption was 0.91 ± 0.67% of the applied dose.
In a 14-day study in five psoriatic patients, measured doses of tazarotene 0.1% gel were applied daily by nursing staff to involved skin without occlusion (8 to 18% of total body surface area; mean ± SD: 13 ± 5%). The Cmax was 12.0 ± 7.6 ng/mL occurring 6 hours after the final dose, and the AUC 0-24hr was 105 ± 55 ng·hr/mL. Systemic absorption was 14.8 ± 7.6% of the applied dose. Extrapolation of these results to represent dosing on 20% of total body surface yielded estimates of Cmax of 18.9 ± 10.6 ng/mL and AUC 0-24hr of 172 ± 88 ng·hr/mL.
An in vitro percutaneous absorption study, using radiolabeled drug and freshly excised human skin or human cadaver skin, indicated that approximately 4 to 5% of the applied dose was in the stratum corneum (tazarotene: AGN 190299 = 5:1) and 2 to 4% was in the viable epidermis-dermis layer (tazarotene: AGN 190299 = 2:1) 24 hours after topical application of the gel.
In two large vehicle-controlled clinical studies, tazarotene 0.05% and 0.1% gels applied once daily for 12 weeks were significantly more effective than vehicle in reducing the severity of the clinical signs of stable plaque psoriasis covering up to 20% of body surface area. In one of the studies, patients were followed up for an additional 12 weeks following cessation of therapy with TAZORAC®. Mean baseline scores and changes from baseline (reductions) after treatment in these two studies are shown in the following table:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||
The 0.1% gel was more effective than the 0.05% gel, but the 0.05% gel was associated with less local irritation than the 0.1% gel (see ADVERSE REACTIONS section).
In two large vehicle-controlled studies, tazarotene 0.1% gel applied once daily was significantly more effective than vehicle in the treatment of facial acne vulgaris of mild to moderate severity. Percent reductions in lesion counts after treatment for 12 weeks in these two studies are shown in the following table:
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
TAZORAC® (tazarotene topical gel) 0.05% and 0.1% are indicated for the topical treatment of patients with stable plaque psoriasis of up to 20% body surface area involvement.
TAZORAC® (tazarotene topical gel) 0.1% is also indicated for the topical treatment of patients with facial acne vulgaris of mild to moderate severity.
The efficacy of TAZORAC® in the treatment of acne previously treated with other retinoids or resistant to oral antibiotics has not been established.
Retinoids may cause fetal harm when administered to a pregnant woman.
In rats, tazarotene 0.05%, administered topically during gestation days 6 through 17 at 0.25 mg/kg/day (1.5 mg/m 2 /day) resulted in reduced fetal body weights and reduced skeletal ossification. Rabbits dosed topically with 0.25 mg/kg/day (2.75 mg/m 2 total body surface area/day) tazarotene during gestation days 6 through 18 were noted with single incidences of known retinoid malformations, including spina bifida, hydrocephaly, and heart anomalies. As with other retinoids, when tazarotene was given orally to experimental animals, developmental delays were seen in rats, and teratogenic effects and post-implantation fetal loss were seen in rats and rabbits at doses producing 0.7 and 13 times, respectively, the systemic exposure (AUC 0-24hr ) in human psoriasis patients, when extrapolated for topical treatment of 20% of body surface area. THUS, SYSTEMIC EXPOSURE IN TOPICALLY TREATED PSORIASIS PATIENTS (FOR USE ON UP TO 20% OF BODY SURFACE AREA) COULD BE IN THE SAME ORDER OF MAGNITUDE AS IN THESE ORALLY TREATED ANIMALS.
Systemic exposure anticipated in the treatment of facial acne may be less, due to a more limited area of application.
Six women inadvertently exposed to TAZORAC® during pregnancy in clinical trials have subsequently delivered healthy babies. As the exact timing and extent of exposure in relation to the gestation time are not certain, the significance of these findings is not known.
TAZORAC® is contraindicated in women who are or may become pregnant. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, treatment should be discontinued and the patient apprised of the potential hazard to the fetus. Women of childbearing potential should be warned of the potential risk and use adequate birth-control measures when TAZORAC® is used. The possibility that a woman of childbearing potential is pregnant at the time of institution of therapy should be considered. A negative result for pregnancy test having a sensitivity down to at least 50 mIU/mL for human chorionic gonadotropin (hCG) should be obtained within 2 weeks prior to TAZORAC® therapy, which should begin during a normal menstrual period.
TAZORAC® is contraindicated in individuals who have shown hypersensitivity to any of its components.
Pregnancy Category X: See CONTRAINDICATIONS section. Women of childbearing potential should be warned of the potential risk and use adequate birth-control measures when TAZORAC® is used. The possibility that a woman of childbearing potential is pregnant at the time of institution of therapy should be considered. A negative result for pregnancy test having a sensitivity down to at least 50 mIU/mL for hCG should be obtained within 2 weeks prior to TAZORAC® therapy, which should begin during a normal menstrual period.
General: TAZORAC® should only be applied to the affected areas. For external use only. Avoid contact with eyes, eyelids, and mouth. If contact with eyes occurs, rinse thoroughly with water. The safety of use over more than 20% of body surface area has not been established in psoriasis or acne.
Retinoids should not be used on eczematous skin, as they may cause severe irritation.
Because of heightened burning susceptibility, exposure to sunlight (including sunlamps) should be avoided unless deemed medically necessary, and in such cases, exposure should be minimized during the use of TAZORAC®. Patients must be warned to use sunscreens (minimum SPF of 15) and protective clothing when using TAZORAC®. Patients with sunburn should be advised not to use TAZORAC® until fully recovered. Patients who may have considerable sun exposure due to their occupation and those patients with inherent sensitivity to sunlight should exercise particular caution when using TAZORAC® and ensure that the precautions outlined in the Information for Patients subsection are observed.
TAZORAC® should be administered with caution if the patient is also taking drugs known to be photosensitizers (e.g., thiazides, tetracyclines, fluoroquinolones, phenothiazines, sulfonamides) because of the increased possibility of augmented photosensitivity.
If pruritus, burning, skin redness or peeling is excessive, the medication should be discontinued until the integrity of the skin is restored.
Weather extremes, such as wind or cold, may be more irritating to patients using TAZORAC®.
Information for Patients: See attached Patient Package Insert.
Drug Interactions: Concomitant dermatologic medications and cosmetics that have a strong drying effect should be avoided. It is also advisable to "rest" a patient' skin until the effects of such preparations subside before use of TAZORAC® is begun.
Carcinogenesis, mutagenesis, impairment of fertility: Long-term studies of tazarotene following oral administration of 0.025, 0.050, and 0.125 mg/kg/day to rats showed no indications of increased carcinogenic risks. However, in other rat studies, oral doses twice that of the highest dose in the rat carcinogenicity study produced an AUC 0-24hr that was less (0.7 times) than that in topically treated psoriatic patients extrapolated for treatment of 20% of body surface area. In evaluation of photocarcinogenicity, median time to onset of tumors was decreased and the number of tumors increased in hairless mice following chronic topical dosing with intercurrent exposure to ultraviolet radiation at tazarotene concentrations of 0.001%, 0.005%, and 0.01% for up to 40 weeks.
A long-term topical application study in mice terminated at 88 weeks showed that dose levels of 0.05, 0.125, 0.25 and 1.0 mg/kg/day (reduced to 0.5 mg/kg/day for males after 41 weeks due to severe dermal irritation) revealed no apparent carcinogenic effects when compared to vehicle control animals; untreated control animals were not completely evaluated. The AUC 0-12hr 's for these doses were 82.7, 137, 183, 136 (males at 1.0/0.5 mg/kg) and 344 ng·hr/mL (females at 1.0 mg/kg), respectively. The mean AUC 0-24hr for psoriatic patients was 172 ng·hr/mL, extrapolated for 20% total body surface area.
Tazarotene was found to be non-mutagenic in the Ames assay and did not produce structural chromosomal aberrations in a human lymphocyte assay. Tazarotene was also non-mutagenic in the CHO/HPRT mammalian cell forward gene mutation assay and was non-clastogenic in the in vivo mouse micronucleus test.
No impairment of fertility occurred in rats when male animals were treated for 70 days prior to mating and female animals were treated for 14 days prior to mating and continuing through gestation and lactation with topical doses of TAZORAC® gel of up to 0.125 mg/kg/day (0.738 mg/m 2 /day).
Reproductive capabilities of F1 animals, including F2 survival and development, were not affected by topical administration of TAZORAC® gel to female F0 parental rats from gestation day 16 through lactation day 20 at the maximum tolerated dose of 0.125 mg/kg/day (0.738 mg/m 2 /day).
Pregnancy: Teratogenic Effects: Pregnancy Category X: See CONTRAINDICATIONS section. Women of childbearing potential should use adequate birth-control measures when TAZORAC® is used. The possibility that a woman of childbearing potential is pregnant at the time of institution of therapy should be considered. A negative result for pregnancy test having a sensitivity down to at least 50 mIU/mL for hCG should be obtained within 2 weeks prior to TAZORAC® therapy, which should begin during a normal menstrual period.
Nursing Mothers: After single topical doses of 14 C-tazarotene to the skin of lactating rats, secretion of radioactivity was detected in milk, suggesting that there would be transfer of drug-related material to the offspring via milk. It is not known whether this drug is excreted in human milk. Caution should be exercised when tazarotene is administered to a nursing woman.
Pediatric Use: The safety and efficacy of tazarotene have not been established in pediatric patients under the age of 12 years.
The most frequent adverse events reported with TAZORAC® 0.05% and 0.1% gels were limited to the skin. Those occurring in 10 to 30% of patients, in descending order, included pruritus, burning/stinging, erythema, worsening of psoriasis, irritation, and skin pain. Events occurring in 1 to 10% of patients included rash, desquamation, irritant contact dermatitis, skin inflammation, fissuring, bleeding and dry skin. Increases in "psoriasis worsening" and "sun-induced erythema" were noted in some patients over the 4th to 12th months as compared to the first three months of a 1 year study. In general, the incidence of adverse events with TAZORAC® 0.05% gel was 2 to 5% lower than that seen with TAZORAC® 0.1% gel.
The most frequent adverse events reported with TAZORAC® 0.1% gel were limited to the skin. Those events occurring in 10 to 30% of patients, in descending order, included desquamation, burning/stinging, dry skin, erythema and pruritus. Events occurring in 1 to 10% of patients included irritation, skin pain, fissuring, localized edema and skin discoloration.
In human dermal safety studies, tazarotene 0.05% and 0.1% gels did not induce contact sensitization, phototoxicity or photoallergy.
Excessive topical use of TAZORAC® may lead to marked redness, peeling, or discomfort (see PRECAUTIONS ).
TAZORAC® is not for oral use. Oral ingestion of the drug may lead to the same adverse effects as those associated with excessive oral intake of Vitamin A (hypervitaminosis A) or other retinoids. If oral ingestion occurs, the patient should be monitored, and appropriate supportive measures should be administered as necessary.
General: Application may cause a transitory feeling of burning or stinging. If irritation is excessive, application should be discontinued.
For psoriasis: Apply TAZORAC® once a day, in the evening, to psoriatic lesions, using enough (2 mg/cm 2 ) to cover only the lesion with a thin film to no more than 20% of body surface area. If a bath or shower is taken prior to application, the skin should be dry before applying the gel. Because unaffected skin may be more susceptible to irritation, application of tazarotene to these areas should be carefully avoided. TAZORAC® was investigated for up to 12 months during clinical trials for psoriasis.
For acne: Cleanse the face gently. After the skin is dry, apply a thin film of TAZORAC® (2 mg/cm 2 ) once a day, in the evening, to the skin where acne lesions appear. Use enough to cover the entire affected area. TAZORAC® was investigated for up to 12 weeks during clinical trials for acne.
TAZORAC® (tazarotene topical gel) is available in concentrations of 0.05% and 0.1%. It comes in collapsible aluminum tubes, in 30 gm and 100 gm sizes.
|
NOTE : TAZORAC® gel should be stored at 25°C (77°F): excursion permitted to 15-30°C (59-86°F).
Rx only
ALLERGAN
Irvine, California 92612, USA December 1998
©1998 Allergan, Inc.
70967 US 12 D