 |
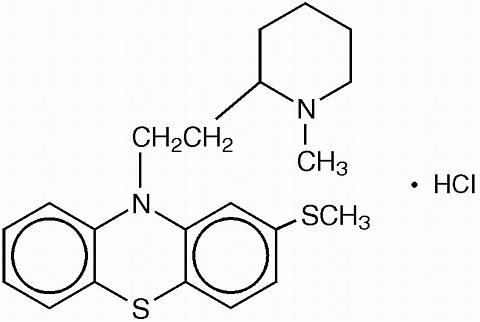
Thioridazine hydrochloride is 10H-Phenothiazine, 10-[2-(1-methyl-2-piperidinyl)ethyl]-2-(methylthio)-, monohydro-chloride. The chemical structure is:
 |
The presence of a thiomethyl radical (S-CH 3 ) in position 2, conventionally occupied by a halogen, is unique and could account for the greater toleration obtained with recommended doses of thioridazine as well as a greater specificity of psychotherapeutic action.
Thioridazine hydrochloride is available as tablets for oral administration containing 10 mg, 25 mg, 50 mg, or 100 mg.
Each tablet for oral administration contains the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, hydroxypropyl cellulose, hydroxypropyl methylcellulose, magnesium stearate, microcrystalline cellulose, polyethylene glylcol, sodium lauryl sulfate, titanium dioxide and FD&C Yellow #6 Aluminum Lake.
Thioridazine hydrochloride is effective in reducing excitement, hypermotility, abnormal initiative, affective tension and agitation through its inhibitory effect on psychomotor functions. Successful modification of such symptoms is the prerequisite for, and often the beginning of the process of recovery in patients exhibiting mental and emotional disturbances.
Thioridazine's basic pharmacological activity is similar to that of other phenothiazines, but certain specific qualities have come to light which support the observation that the clinical spectrum of this drug shows significant differences from those of the other agents of this class. Minimal anti-emetic activity and minimal extrapyramidal stimulation, notably pseudo-parkinsonism, are distinctive features of this drug.
For the management of manifestations of psychotic disorders.
For the short-term treatment of moderate to marked depression with variable degrees of anxiety in adult patients and for the treatment of multiple symptoms such as agitation, anxiety, depressed mood, tension, sleep disturbances, and fears in geriatric patients.
For the treatment of severe behavioral problems in children marked by combativeness and/or explosive hyperexcitable behavior (out of proportion to immediate provocations), and in the short-term treatment of hyperactive children who show excessive motor activity with accompanying conduct disorders consisting of some or all of the following symptoms: impulsivity, difficulty sustaining attention, aggressivity, mood lability, and poor frustration tolerance.
In common with other phenothiazines, thioridazine hydrochloride is contraindicated in severe central nervous system depression or comatose states from any cause. It should also be noted that hypertensive or hypotensive heart disease of extreme degree is a contraindication of phenothiazine administration.
Tardive Dyskinesia: Tardive dyskinesia, a syndrome consisting of potentially irreversible, involuntary, dyskinetic movements may develop in patients treated with neuroleptic (antipsychotic) drugs. Although the prevalence of the syndrome appears to be highest among the elderly, especially elderly women, it is impossible to rely upon prevalence estimates to predict, at the inception of neuroleptic treatment, which patients are likely to develop the syndrome. Whether neuroleptic drug products differ in their potential to cause tardive dyskinesia is unknown.
Both the risk of developing the syndrome and the likelihood that it will become irreversible are believed to increase as the duration of treatment and the total cumulative dose of neuroleptic drugs administered to the patients increase. However, the syndrome can develop, although much less commonly, after relatively brief treatment periods at low doses.
There is no known treatment for established cases of tardive dyskinesia, although the syndrome may remit, partially or completely, if neuroleptic treatment is withdrawn. Neuroleptic treatment, itself, however, may suppress (or partially suppress) the signs and symptoms of the syndrome and thereby may possibly mask the underlying disease process. The effect that symptomatic suppression has upon the long-term course of the syndrome is unknown.
Given these considerations, neuroleptics should be prescribed in a manner that is most likely to minimize the occurrence of tardive dyskinesia. Chronic neuroleptic treatment should generally be reserved for patients who suffer from a chronic illness that, 1) is known to respond to neuroleptic drugs, and, 2) for whom alternative, equally effective, but potentially less harmful treatments are not available or appropriate. In patients who do require chronic treatment, the smallest dose and the shortest duration of treatment producing a satisfactory clinical response should be sought. The need for continued treatment should be re-assessed periodically.
If signs and symptoms of tardive dyskinesia appear in a patient on neuroleptics, drug discontinuation should be considered. However, some patients may require treatment despite the presence of the syndrome.
(For further information about the of tardive dyskinesia and its clinical detection, please refer to the sections on Information for Patients and ADVERSE REACTIONS ).
Neuroleptic Malignant Syndrome (NMS): A potentially fatal symptom complex sometimes referred to as Neuroleptic Malignant Syndrome (NMS) has been reported in association with antipsychotic drugs. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmias).
The diagnostic evaluation of patients with this syndrome is complicated. In arriving at a diagnosis, it is important to identify cases where the clinical presentation includes both serious medical illness (e.g., pneumonia, systemic infection, etc.) and untreated or inadequately treated extrapyramidal signs and symptoms (EPS). Other important considerations in the differential diagnosis include central anticholinergic toxicity, heat stroke, drug fever and primary central nervous system (CNS) pathology.
The management of NMS should include 1) immediate discontinuation of antipsychotic drugs and other drugs not essential to concurrent therapy, 2) intensive symptomatic treatment and medical monitoring, and 3) treatment of any concomitant serious medical problems for which specific treatments are available. There is no general agreement about specific pharmacological treatment regimens for uncomplicated NMS.
If the patient requires anti-psychotic drug treatment after recovery from NMS, the potential reintroduction of drug therapy should be carefully considered. The patient should be carefully monitored, since recurrences of NMS have been reported.
General: It has been suggested in regard to phenothiazines in general, that people who have demonstrated a hypersensitivity reaction (e.g., blood dyscrasias, jaundice) to one may be more prone to demonstrate a reaction to others. Attention should be paid to the fact that phenothiazines are capable of potentiating central nervous system depressants (e.g., anesthetics, opiates, alcohol, etc.) as well as atropine and phosphorus insecticides. Physicians should carefully consider benefit versus risk when treating less severe disorders.
Reproductive studies in animals and clinical experience to date have failed to show a teratogenic effect with thioridazine. However, in view of the desirability of keeping the administration of all drugs to a minimum during pregnancy, thioridazine should be given only when the benefits derived from treatment exceed the possible risks to mother and fetus.
Leukopenia and/or agranulocytosis and convulsive seizures have been reported but are infrequent. Thioridazine has been shown to be helpful in the treatment of behavioral disorders in epileptic patients, but anticonvulsant medication should also be maintained. Pigmentary retinopathy, which has been observed primarily in patients taking larger than recommended doses, is characterized by diminution of visual acuity, brownish coloring of vision, and impairment of night vision; examination of the fundus discloses deposits of pigment. The possibility of this complication may be reduced by remaining within the recommended limits of dosage.
Where patients are participating in activities requiring complete mental alertness (e.g., driving) it is advisable to administer the phenothiazines cautiously and to increase the dosage gradually. Female patients appear to have a greater tendency to orthostatic hypotension than male patients. The administration of epinephrine should be avoided in the treatment of drug-induced hypotension in view of the fact that phenothiazines may induce a reversed epinephrine effect on occasion. Should a vasoconstrictor be required, the most suitable are norepinephrine and phenylephrine.
Neuroleptic drugs elevate prolactin levels; the elevation persists during chronic administration. Tissue culture experiments indicate that approximately one-third of human breast cancers are prolactin dependent in vitro , a factor of potential importance if the prescription of these drugs is contemplated in a patient with a previously detected breast cancer. Although disturbances such as galactorrhea, amenorrhea, gynecomastia, and impotence have been reported, the clinical significance of elevated serum prolactin levels is unknown for most patients. An increase in mammary neoplasms has been found in rodents after chronic administration of neuroleptic drugs. Neither clinical studies nor epidemiologic studies conducted to date, however, have shown an association between chronic administration of these drugs and mammary tumorigenesis; the available evidence is considered too limited to be conclusive at this time.
Concurrent administration of propranolol (100 mg and 800 mg daily) has been reported to produce increases in plasma levels of thioridazine (approximately 50 to 400 percent) and its metabolites (approximately 80 to 300 percent).
It is recommended that a daily dose in excess of 300 mg be reserved for use only in severe neuropsychiatric conditions.
Information for Patients: Given the likelihood that some patients exposed chronically to neuroleptics will develop tardive dyskinesia, it is advised that all patients in whom chronic use is contemplated be given, if possible, full information about this risk. The decision to inform patients and/or their guardians must obviously take into account the clinical circumstances and the competency of the patient to understand the information provided.
In the recommended dosage ranges with thioridazine hydrochloride most side effects are mild and transient.
Central Nervous System: Drowsiness may be encountered on occasion, especially where large doses are given early in treatment. Generally, this effect tends to subside with continued therapy or a reduction in dosage. Pseudo-parkinsonism and other extrapyramidal symptoms may occur but are infrequent. Nocturnal confusion, hyperactivity, lethargy, psychotic reactions, restlessness and headache have been reported but are extremely rare.
Autonomic Nervous System: Dryness of mouth, blurred vision, constipation, nausea, vomiting, diarrhea, nasal stuffiness and pallor have been seen.
Endocrine System: Galactorrhea, breast engorgement, amenorrhea, inhibition of ejaculation and peripheral edema have been described.
Skin Dermatitis and skin eruptions of the urticarial type have been observed infrequently. Photosensitivity is extremely rare.
Cardiovascular System: ECG changes have been reported (see Phenothiazine Derivatives : Cardiovascular Effects ).
Other: Rare cases described as parotid swelling have been reported following administration of thioridazine.
Phenothiazine Derivatives: It should be noted that efficacy, indications and untoward effects have varied with the different phenothiazines. It has been reported that old age lowers the tolerance for phenothiazines. The most common neurological side effects in these patients are parkinsonism and akathisia. There appears to be an increased risk of agranulocytosis and leukopenia in the geriatric population. The physician should be aware that the following have occurred with one or more phenothiazines and should be considered whenever one of these drugs is used.
Autonomic Reactions: Miosis, obstipation, anorexia, paralyltic ileus.
Cutaneous Reactions: Erythema, exfoliative dermatitis, contact dermatitis.
Blood Dyscrasias: Agranulocytosis, leukopenia, eosinophilia, thrombocytopenia, anemia, aplastic anemia, pancytopenia.
Allergic Reactions: Fever, laryngeal edema, angioneurotic edema, asthma.
Hepatotoxicity: Jaundice, biliary stasis.
Cardiovascular Effects: Changes in the terminal portion of the electrocardiogram, including prolongation of the Q-T interval, lowering and inversion of the T-wave and appearance of a wave tentatively identified as a bifid T or a U wave have been observed in some patients receiving the phenothiazine tranquilizers, including thioridazine hydrochloride. To date, these appear to be due to altered repolarization and not related to myocardial damage. They appear to be reversible. While there is no evidence at present that these changes are in any way precursors of any significant disturbance of cardiac rhythm, it should be noted that several sudden and unexpected deaths apparently due to cardiac arrest have occurred in patients previously showing characteristic electrocardiographic changes while taking the drug. The use of periodic electrocardiograms has been proposed but would appear to be of questionable value as a predictive device. Hypotension, rarely resulting in cardiac arrest.
Extrapyramidal Symptoms: Akathisia, agitation, motor restlessness, dystonic reactions, trismus, torticollis, opisthotonus, oculogyric crises, tremor, muscular rigidity, akinesia.
Tardive Dyskinesia: Chronic use of neuroleptics may be associated with the development of tardive dyskinesia. The salient features of this syndrome are described in the section and below.
The syndrome is characterized by involuntary choreoathetoid movements which variously involve the tongue, face, mouth, lips, or jaw (e.g. protrusion of the tongue, puffing of cheeks, puckering of the mouth, chewing movements), trunk and extremities. The severity of the syndrome and the degree of impairment produced vary widely.
The syndrome may become clinically recognizable either during treatment, upon dosage reduction, or upon withdrawal of treatment. Movements may decrease in intensity and may disappear altogether if further treatment with neuroleptics is withheld. It is generally believed that reversibility is more likely after short rather than long-term neuroleptic exposure. Consequently, early detection of tardive dyskinesia is important. To increase the likelihood of detecting the syndrome at the earliest possible time, the dosage of neuroleptic drug should be reduced periodically (if clinically possible) and the patient observed for signs of the disorder. This maneuver is critical, for neuroleptic drugs may mask the signs of the syndrome.
Endocrine Disturbances: Menstrual irregularities, altered libido, gynecomastia, lactation, weight gain, edema. False positive pregnancy tests have been reported.
Urinary Disturbances: Retention, incontinence.
Others: Hyperpyrexia. Behavioral effects suggestive of a paradoxical reaction have been reported. These include excitement, bizarre dreams, aggravation of psychoses and toxic confusional states. More recently, a peculiar skin-eye syndrome has been recognized as a side effect following long-term treatment with phenothiazines. This reaction is marked by progressive pigmentation of areas of the skin or conjunctiva and/or accompanied by discoloration of the exposed sclera and cornea. Opacities of the anterior lens and cornea described as irregular or stellate in shape have also been reported. Systemic lupus erythematosus-like syndrome.
Dosage must be individualized according to the degree of mental and emotional disturbance. In all cases, the smallest effective dosage should be determined for each patient.
Adults: Psychotic manifestations: The usual starting dose is 50 to 100 mg three times a day, with a gradual increment to a maximum of 800 mg daily if necessary. Once effective control of symptoms has been achieved, the dosage may be reduced gradually to determine the minimum maintenance dose. The total daily dosage ranges from 200 to 800 mg divided into two to four doses.
For the short-term treatment of moderate to marked depression with variable degrees of anxiety in adult patients and for the treatment of multiple symptoms such as agitation, anxiety, depressed mood, tension, sleep disturbances, and fears in geriatric patients:
The usual starting dose is 25 mg three times a day. Dosage ranges from 10 mg two to four times a day in milder cases to 50 mg three or four times a day for more severely disturbed patients. The total daily dosage range is from 20 mg to a maximum of 200 mg.
Children: Thioridazine is not intended for children under 2 years of age. For children aged 2 to 12 the dosage of thioridazine hydrochloride ranges from 0.5 mg to a maximum of 3 mg/kg per day. For children with moderate disorders 10 mg two or three times a day is the usual starting dose. For hospitalized, severely disturbed, or psychotic children, 25 mg two or three times daily is the usual starting dose. Dosage may be increased gradually until optimum therapeutic effect is obtained or the maximum has been reached.
Thioridazine Hydrochloride Tablets, USP are available containing 10 mg, 25 mg, 50 mg, or 100 mg of thioridazine hydrochloride.
The 10 mg tablets are orange, round, unscored film coated tablets marked with M54 on one side and 10 on the other side. They are available as follows:
NDC 0378-0612-01 bottles of 100 tablets
NDC 0378-0612-10 bottles of 1000 tablets
The 25 mg tablets are orange, round, unscored film coated tablets marked with M58 on one side and 25 on the other side. They are available as follows:
NDC 0378-0614-01 bottles of 100 tablets
NDC 0378-0614-10 bottles of 1000 tablets
The 50 mg tablets are orange, round, unscored film coated tablets marked with M59 on one side and 50 on the other side. They are available as follows:
NDC 0378-0616-01 bottles of 100 tablets
NDC 0378-0616-10 bottles of 1000 tablets
The 100 mg tablets are orange, round, unscored film coated tablets marked with M61 on one side and 100 on the other side. They are available as follows:
NDC 0378-0618-01 bottles of 100 tablets
NDC 0378-0618-10 bottles of 1000 tablets
STORE AT CONTROLLED ROOM TEMPERATURE 15°-30°C (59°-86°F).
PROTECT FROM LIGHT.
Dispense in a tight, light-resistant container using a child-resistant closure.
MYLAN®
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505
REVISED MARCH 1998
THIO:R7AQ