 |
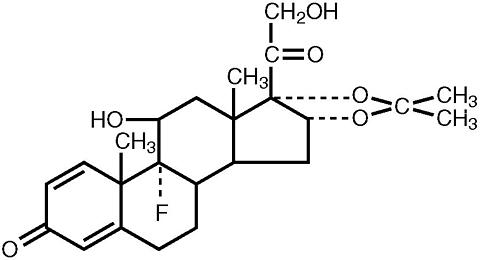
Triamcinolone acetonide, the active ingredient of Tri-Nasal® Spray , is a corticosteroid with the chemical name, 9(alpha)-Fluoro-11(beta),16(alpha), 17, 21-tetrahydroxypregna-1,4-diene-3, 20-dione cyclic 16, 17-acetal with acetone (C 24 H 31 FO 6 ). Its structural formula is:
 |
Triamcinolone acetonide, USP, is a white crystalline powder, with a molecular weight of 434.51. It is practically insoluble in water, and sparingly soluble in dehydrated alcohol, in chloroform and in methanol. It has a melting point temperature range between 292° and 294°C.
Tri-Nasal® Spray is a metered-dose manual spray pump in an amber polyethylene terephthalate (PET) bottle with 0.05% w/v triamcinolone acetonide in a solution containing citric acid, edetate disodium, polyethylene glycol 3350, propylene glycol, purified water, sodium citrate, and 0.01% benzalkonium chloride as a preservative. Tri-Nasal® Spray pH is 5.3.
After initial priming (three sprays) of the Tri-Nasal® Spray metered pump delivery system, each spray will deliver 50 mcg of triamcinolone acetonide. If the pump was not used for more than 14 days, reprime with 3 sprays or until a fine mist is observed. The majority of the droplets produced by the pump are 8 microns or greater. Each 15 mL bottle contains 7.5 mg of triamcinolone acetonide to deliver 120 metered sprays. After 120 sprays, the amount of triamcinolone acetonide delivered per spray may not be consistent and the bottle should be discarded.
Triamcinolone acetonide is a more potent derivative of triamcinolone. Triamcinolone acetonide is approximately eight times more potent than prednisone in animal models of inflammation.
Although the precise mechanism of corticosteroid antiallergic action is unknown, corticosteroids are very effective. When allergic symptoms are very severe, local treatment with recommended doses (microgram) of available topical corticosteroids are not as effective as treatment with larger doses (milligram) of oral or parenteral formulations.
Absorption : The pharmacokinetics of Tri-Nasal® Spray was evaluated in a single-dose study conducted in 24 patients with perennial allergic rhinitis. Following a single intranasal dose of 400 mcg of triamcinolone acetonide (twice the recommended starting dose of Tri-Nasal® Spray ), the mean C max of the drug was 1.12 ng/mL (SD = 0.38) with a median T max of 0.5 hours (range: 0.08 - 1.0).
A pharmacokinetic study to demonstrate dose proportionality was conducted in patients with perennial allergic rhinitis. The C max and AUC of the 200 and 400 mcg doses increased less than proportionally when compared to the 100 mcg dose. Following multiple dosing (100 or 200 or 400 mcg QD for 7 days), there was no evidence of drug accumulation.
Distribution : The volume of distribution (Vd) reported was 99.5 L (SD = 27.5).
Metabolism : In animal studies using rats and dogs, three metabolites of triamcinolone acetonide have been identified. They are 6(beta)-hydroxytriamcinolone acetonide, 21-carboxytriamcinolone acetonide and 21-carboxy-6(beta)-hydroxytriamcinolone acetonide. All three metabolites are expected to be substantially less active than the parent compound due to (a) the dependence of anti-inflammatory activity on the presence of a 21-hydroxyl group, (b) the decreased activity observed upon 6-hydroxylation, and (c) the markedly increased water solubility favoring rapid elimination. There appeared to be some quantitative differences in the metabolites among species. No differences were detected in metabolic pattern as a function of route of administration.
Elimination : After a single intranasal dose of 400 mcg of triamcinolone acetonide (twice the recommended starting dose of Tri-Nasal® Spray ), the mean observed elimination half-life was 2.26 hours (SD=0.77). Based upon intravenous dosing of triamcinolone acetonide phosphate ester, the half-life of triamcinolone acetonide was reported to be 88 minutes. The reported clearance was 45.2 L/hour (SD=9.1) for triamcinolone acetonide.
Age : The effect of age, specifically in geriatric and pediatric patients, on the pharmacokinetics of triamcinolone acetonide has not been studied.
Gender : Gender did not significantly influence the pharmacokinetics of Tri-Nasal® Spray .
Race : The effect of race on the pharmacokinetics of Tri-Nasal® Spray has not been studied.
Renal/Hepatic Insufficiency : No specific pharmacokinetic studies have been conducted in renally or hepatically impaired subjects.
Drug-Drug Interactions : No specific drug-drug interactions have been investigated.
A small (approximately 5 to 7 patients per treatment group), parallel trial was conducted to assess the effect of Tri-Nasal® Spray on the Hypothalamic-Pituitary-Adrenal (HPA) axis. Patients with allergic rhinitis were treated for six weeks with 400 mcg, 800 mcg, or 1600 mcg total daily doses of Tri-Nasal® Spray , 10 mg oral prednisone once daily, or placebo. Adrenal response to a six-hour cosyntropin stimulation test suggests that intranasal Tri-Nasal® Spray 400 mcg/day for six weeks did not measurably affect adrenal activity. Tri-Nasal® treatment arms using doses of 800 and 1600 mcg/day demonstrated a trend toward dose-related suppression of HPA response. However, this decrease did not reach statistical significance, whereas 10 mg daily oral prednisone did.
The efficacy of Tri-Nasal® Spray has been evaluated in 746 patients with seasonal or perennial allergic rhinitis who completed 8 controlled clinical trials.
In total, 1187 patients have been treated with Tri-Nasal® Spray in the clinical development program. Three adequate and well controlled multi-center trials involving 541 patients with seasonal allergic rhinitis who received doses of Tri-Nasal® Spray ranging from 50 mcg to 400 mcg once daily were conducted. The results showed that patients who received >/= 200 mcg daily of the active drug had statistically significant relief in the severity of nasal symptoms of seasonal allergic rhinitis including sneezing, stuffiness, discharge, and itching, compared to those receiving placebo.
In one clinical trial that examined efficacy after 2 days of 200 or 400 mcg Tri-Nasal® Spray treatment, only the 400 mcg dose showed statistically significant improvement over placebo in the nasal symptoms of seasonal allergic rhinitis.
Tri-Nasal® Spray is indicated for the treatment of the nasal symptoms of seasonal and perennial allergic rhinitis in adults and children 12 years of age or older.
Hypersensitivity to any of the ingredients of this preparation contraindicates its use.
The replacement of a systemic corticosteroid with a topical corticosteroid can be accompanied by signs of adrenal insufficiency and, in addition, some patients may experience symptoms of corticosteroid withdrawal, e.g., joint and/or muscular pain, lassitude and depression. Patients previously treated for prolonged periods with systemic corticosteroids and transferred to Tri-Nasal® Spray should be carefully monitored for acute adrenal insufficiency in response to stress. In those patients who have asthma or other clinical conditions which require long-term corticosteroid treatment, too rapid a decrease in systemic corticosteroid may cause a severe exacerbation of their symptoms.
Persons who are on immunosuppressant drugs are more susceptible to infections than healthy individuals. Chickenpox and measles, for example, can have a more serious or even fatal course in children or adults on immunosuppressant doses of corticosteroids. In such children or adults who have not had these diseases, particular care should be taken to avoid exposure. If exposed, therapy with varicella zoster immune globulin (VZIG) or pooled intravenous immunoglobulin (IVIG) as appropriate, may be indicated. If chickenpox develops, treatment with antiviral agents may be considered.
General: Intranasal corticosteroids may cause a reduction in growth velocity when administered to pediatric patients (see PRECAUTIONS , Pediatric Use section).
In clinical studies with triamcinolone acetonide nasal spray, the development of localized infections of the nose and pharynx with Candida albicans has rarely occurred. When such an infection develops it may require treatment with appropriate local therapy and discontinuance of treatment with Tri-Nasal® Spray .
Tri-Nasal® Spray should be used with caution, if at all, in patients with active or quiescent tuberculous infection of the respiratory tract or in patients with untreated fungal, bacterial, or systemic viral infections or ocular herpes simplex.
Because of the inhibitory effect of corticosteroids on wound healing, in patients who have experienced recent nasal septal ulcers, nasal surgery or trauma, a corticosteroid should be used with caution until healing has occurred. As with other nasally inhaled corticosteroids, nasal septal perforations have been reported in rare instances.
When used at excessive doses, systemic corticosteroid effects such as hypercorticism and adrenal suppression may appear. If such changes occur, Tri-Nasal® Spray should be discontinued slowly, consistent with accepted procedures for discontinuing oral corticosteroid therapy.
Systemic Availability and HPA Axis Suppression: Triamcinolone acetonide administered intranasally as Tri-Nasal® Spray has been shown to be absorbed into the systemic circulation in humans. The bioavailability of triamcinolone acetonide when administered as a solution in Tri-Nasal® Spray is approximately 5-fold greater than when administered as a CFC aerosol suspension formulation. While Tri-Nasal® Spray administered to 5 patients with allergic rhinitis at 400 mcg/day for 42 days did not measurably affect adrenal response to a six-hour cosyntropin stimulation test, the 6-hour cosyntropin test is an insensitive assessment for subtle HPA effects of corticosteroids. Doses of 800 and 1600 mcg/day of Tri-Nasal® Spray did demonstrate a trend toward dose-related suppression of the HPA response. However, this decrease did not reach statistical significance, whereas 10 mg daily oral prednisone did. (see , Pharmacodynamics )
Patients being treated with Tri-Nasal® Spray should receive the following information and instructions:
Carcinogenesis, Mutagenesis and Impairment of Fertility: In two-year mouse and Sprague-Dawley rat studies, triamcinolone acetonide did not increase the incidence of tumors at oral doses up to 1 and 3 mcg/kg, respectively (less than the maximum recommended daily intranasal dose on a mcg/m 2 basis
The genotoxic potential of triamcinolone acetonide has not been studied.
Triamcinolone acetonide did not impair fertility in Sprague-Dawley rats given oral doses up to 15 mcg/kg (less than the maximum recommended daily intranasal dose on a mcg/m 2 basis
However, triamcinolone acetonide caused increased fetal resorptions and stillbirths and decreased pup weight and survival at 5 mcg/kg (less than the maximum recommended daily intranasal dose on a mcg/m 2 basis). These effects were not produced at 1 mcg/kg (less than the maximum recommended daily intranasal dose on a mcg/m 2 basis
Pregnancy Pregnancy Category C.
Triamcinolone acetonide induced cleft palate, internal hydrocephaly and skeletal defects in fetuses of Sprague-Dawley rats and New Zealand White rabbits treated throughout organogenesis with daily inhalation doses of 20 mcg/kg (less than the maximum recommended daily intranasal dose on a mcg/m 2 basis). Triamcinolone acetonide induced cranial malformations in fetuses of Rhesus monkeys treated throughout organogenesis with daily intramuscular doses of 500 mcg/kg and greater (approximately 20 times the maximum recommended daily intranasal dose on a mcg/m 2 basis). The 500 mcg/kg dose was the lowest dose used in this study.
There are no adequate and well-controlled studies in pregnant women. Triamcinolone acetonide should be used during pregnancy only if the potential benefits justify the potential risk to the fetus. Since their introduction, experience with oral corticosteroids in pharmacologic as opposed to physiologic doses suggests that rodents are more prone to teratogenic effects from corticosteroids than humans. In addition, because there is a natural increase in corticosteroid production during pregnancy, most women will require a lower exogenous corticosteroid dose and many will not need corticosteroid treatment during pregnancy.
Nonteratogenic Effects: Hypoadrenalism may occur in infants born of mothers receiving corticosteroids during pregnancy. Such infants should be carefully observed.
Nursing Mothers: It is not known whether triamcinolone acetonide is excreted in human breast milk. Because other corticosteroids are excreted in human milk, caution should be exercised when Tri-Nasal® Spray is administered to nursing women.
Pediatric Use: Controlled clinical studies have shown that intranasal corticosteroids may cause a reduction in growth velocity in pediatric patients. This effect has been observed in the absence of laboratory evidence of hypothalamic-pituitary-adrenal (HPA) axis suppression, suggesting that growth velocity is a more sensitive indicator of systemic corticosteroid exposure in pediatric patients than some commonly used tests of HPA axis function. The long-term effects of this reduction in growth velocity associated with intranasal corticosteroids, including the impact on final adult height, are unknown. The potential for "catch up" growth following discontinuation of treatment with intranasal corticosteroids has not been adequately studied. The growth of pediatric patients receiving intranasal corticosteroids, including Tri-Nasal® Spray should be monitored routinely (e.g. via stadiometry). The potential growth effects of prolonged treatment should be weighed against clinical benefits obtained and the availability of safe and effective noncorticosteroid treatment alternatives. To minimize the systemic effects of intranasal corticosteroids, including Tri-Nasal® Spray , each patient should be titrated to the lowest dose that effectively controls his/her symptoms.
In adequate, well-controlled and uncontrolled studies, 1187 patients have received Tri-Nasal® Spray . The adverse reactions summarized below, are based upon seven placebo controlled clinical trials of 2-6 weeks duration in 847 patients with seasonal or perennial allergic rhinitis (504 patients received 200 mcg or 400 mcg per day of Tri-Nasal® Spray and 343 patients received vehicle placebo). Adverse events reported by 2% or more of patients (regardless of relationship to treatment) who received Tri-Nasal® Spray 200 or 400 mcg once daily and that were more common with Tri-Nasal® Spray than with placebo are displayed in the table below. Overall, the incidence and nature of adverse events with Tri-Nasal® 400 mcg was comparable to that seen with Tri-Nasal® 200 mcg and with vehicle placebo.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Adverse events reported by 2% or more of patients who received Tri-Nasal® Spray 200 or 400 mcg once daily and that were more common with placebo than with Tri-Nasal® Spray included: application site reaction (e.g. transient nasal burning and stinging), rhinitis, dysmenorrhea, pain (unspecified) and allergic reaction.
The adverse effects related to the irritation of nasal mucous membranes (i.e. application site reaction) did not usually interfere with treatment. In the controlled and uncontrolled studies, approximately 0.3% of patients discontinued because of irritation of nasal mucous membranes.
In the event of accidental overdose, an increased potential for these adverse experiences may be expected, but systemic adverse experiences are unlikely.
(see OVERDOSAGE section
Cases of growth suppression have been reported for intranasal corticosteroids. (see PRECAUTIONS , Pediatric Use section).
Like any other nasally administered corticosteroid, acute overdosage is unlikely. The acute topical application of the entire 15 mL of the bottle would most likely cause nasal irritation and headache. Significant acute systemic adverse effects are unlikely even if the entire 7.5 mg of triamcinolone acetonide is administered intranasally at one time. The intranasal median lethal dose has not been determined in animals.
The usual recommended starting dose of Tri-Nasal® Spray for most patients is 200 mcg per day given as 2 sprays (approximately 50 mcg/spray) in each nostril once a day. The maximum dose should not exceed 400 mcg per day. If the 400 mcg dose is used, it may be given either as a once a day dosage (4 sprays in each nostril) or divided into two daily doses of two sprays/nostril twice a day.
The nasal spray pump must be primed before Tri-Nasal® Spray is used for the first time. To prime the pump, press down on the shoulder of the white nasal applicator using your forefinger and middle finger while supporting the base of the bottle with your thumb. Press down and release the pump until it sprays 3 times or until a fine mist is observed (see DIRECTIONS FOR USE ).
Dosing of Tri-Nasal® Spray should be individualized since there are many variables that determine clinical response. These variables include the degree of patient allergy and degree of pollen exposure, both of which may influence the dose required.
A starting dose of 200 mcg (2 sprays/nostril) once daily is recommended for most patients.
If the patient does not receive a satisfactory response from the initial 200 mcg/day starting dose, the dose may be increased to a maximum of 400 mcg (4 sprays/nostril) once daily. An alternative 400 mcg per day dosing regimen may be given as 200 mcg twice daily (two 50 mcg sprays in each nostril twice daily).
Some patients may obtain relief of symptoms sooner when started on a 400 mcg per day dose of Tri-Nasal® Spray than with 200 mcg per day. Onset of significant relief of nasal symptoms was seen within two days after starting treatment at 400 mcg once daily. A starting dose of 400 mcg per day may be considered in patients when starting therapy with Tri-Nasal® Spray in cases where a faster onset of relief is desirable. Generally, maximum relief of symptoms may take several days or up to one week to occur.
After symptoms have been brought under control, patients should be titrated to the minimum effective dose to reduce the possibility of adverse effects.
If relief of symptoms is not achieved after 14-21 days of Tri-Nasal® Spray therapy given in an adequate dose, Tri-Nasal® Spray should be discontinued and alternative diagnosis and therapies considered.
The maximum daily dose should not exceed 400 mcg. (see PRECAUTIONS , , INFORMATION FOR PATIENTS and ADVERSE REACTIONS sections).
Tri-Nasal® Spray is not recommended for use in persons under 12 years of age since its safety and effectiveness have not been established in this age group.
Directions for Use: Illustrated patient instructions for use accompany each package of Tri-Nasal® Spray .
Each 15 mL bottle of Tri-Nasal® Spray (NDC# 0451-5050-15) contains 7.5 mg (0.50 mg/mL) of triamcinolone acetonide, USP and is fitted with a meter pump with white nasal applicator, teal blue dust cover and teal blue locking clip sealed in a foil pouch. The unit delivers 120 metered sprays and comes with a patient' instructions for use leaflet.
Do not spray in eyes.
Store at controlled room temperature: 20°-25°C (68°-77°F). Protect from freezing.
Use Tri-Nasal® Spray within 3 months after opening of the protective foil pouch or before expiration date, whichever comes first.
Rx only
Muro
Pharmaceutical, Inc.
an ASTA Medica company
Tewksbury, MA 01876
I-5050 Revision 2/2000
0000496