 |
URSO® is a bile acid available as 250 mg film-coated tablets for oral administration.
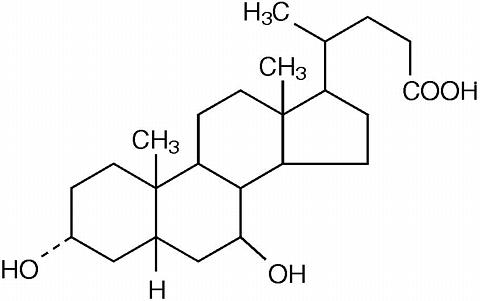
URSO® is ursodiol (ursodeoxycholic acid), a naturally occurring bile acid found in small quantities in normal human bile and in larger quantities in the biles of certain species of bears. It is a bitter-tasting white powder consisting of crystalline particles freely soluble in ethanol and glacial acetic acid, slightly soluble in chloroform, sparingly soluble in ether, and practically insoluble in water. The chemical name of ursodiol is 3(alpha),7(beta)-dihydroxy-5(beta)-cholan-24-oic (C 24 H 40 O 4 ). Ursodiol has a molecular weight of 392.56. Its structure is shown below.
 |
Inactive ingredients: microcrystalline cellulose, povidone, sodium starch glycolate, magnesium stearate, ethylcellulose, dibutyl sebacate, carnauba wax, hydroxypropyl methylcellulose, PEG 3350, PEG 8000, cetyl alcohol, sodium lauryl sulfate and hydrogen peroxide.
Ursodiol (UDCA) is normally present as a minor fraction of the total bile acids in humans (about 5%). Following oral administration, the majority of ursodiol is absorbed by passive diffusion and its absorption is incomplete. Once absorbed, ursodiol undergoes hepatic extraction to the extent of about 50% in the absence of liver disease. As the severity of liver disease increases, the extent of extraction decreases. In the liver, ursodiol is conjugated with glycine or taurine, then secreted into bile. These conjugates of ursodiol are absorbed in the small intestine by passive and active mechanisms. The conjugates can also be deconjugated in the ileum by intestinal enzymes, leading to the formation of free ursodiol that can be reabsorbed and reconjugated in the liver. Nonabsorbed ursodiol passes into the colon where it is mostly 7-dehydroxylated to lithocholic acid. Some ursodiol is epimerized to chenodiol (CDCA) via a 7-oxo intermediate. Chenodiol also undergoes 7-dehydroxylation to form lithocholic acid. These metabolites are poorly soluble and excreted in the feces. A small portion of lithocholic acid is reabsorbed, conjugated in the liver with glycine, or taurine and sulfated at the 3 position. The resulting sulfated lithocholic acid conjugates are excreted in bile and then lost in feces.
Lithocholic acid, when administered chronically to animals, causes cholestatic liver injury that may lead to death from liver failure in certain species unable to form sulfate conjugates. Ursodiol is 7-dehydroxylated more slowly than chenodiol. For equimolar doses of ursodiol and chenodiol, steady state levels of lithocholic acid in biliary bile acids are lower during ursodiol administration than with chenodiol administration. Humans and chimpanzees can sulfate lithocholic acid. Although liver injury has not been associated with ursodiol therapy, a reduced capacity to sulfate may exist in some individuals. Nonetheless, such a deficiency has not yet been clearly demonstrated and must be extremely rare, given the several thousand patient-years of clinical experience with ursodiol.
In healthy subjects, at least 70% of ursodiol (unconjugated) is bound to plasma protein. No information is available on the binding of conjugated ursodiol to plasma protein in healthy subjects or primary biliary cirrhosis (PBC) patients. Its volume of distribution has not been determined, but is expected to be small since the drug is mostly distributed in the bile and small intestine. Ursodiol is excreted primarily in the feces. With treatment, urinary excretion increases, but remains less than 1% except in severe cholestatic liver disease.
During chronic administration of ursodiol, it becomes a major biliary and plasma bile acid. At a chronic dose of 13-15 mg/kg/day, ursodiol constitutes 30-50% of biliary and plasma bile acids.
A U.S., multicenter, randomized, double-blind, placebo-controlled study was conducted to evaluate the efficacy of ursodeoxycholic acid at a dose of 13-15 mg/kg/day, administered in 4 divided doses in 180 patients with PBC. Upon completion of the double-blind portion, all patients entered an open-label active treatment extension phase.
Treatment failure, the main efficacy end point measured during this study, was defined as death, need for liver transplantation, histologic progression by two stages or to cirrhosis, development of varices, ascites or encephalopathy, marked worsening of fatigue or pruritus, inability to tolerate the drug, doubling of serum bilirubin and voluntary withdrawal. After two years of double-blind treatment, the incidence of treatment failure was significantly reduced in the URSO® group (n=89) as compared to the placebo group (n=91). Time to treatment failure was also significantly delayed in the URSO® treated group regardless of either histologic stage or baseline bilirubin levels (>1.8 or </=1.8 mg/dl).
Using a definition of treatment failure which excluded doubling of serum bilirubin and voluntary withdrawal, time to treatment failure was significantly delayed in the URSO® group. In comparison with placebo, treatment with URSO® resulted in a significant improvement in the following serum hepatic biochemistries when compared to baseline: total bilirubin, SGOT, alkaline phosphatase and IgM.
A second study conducted in Canada randomized 222 PBC patients to ursodiol, 14 mg/kg/day or placebo, in a double-blind manner during a two-year period. At two years, a statistically significant difference between the two treatments, in favor of ursodiol, was demonstrated in the following: reduction in the proportion of patients exhibiting a more than 50% increase in serum bilirubin; median percent decrease in bilirubin, transaminases and alkaline phosphatase; incidence of treatment failure; and time to treatment failure. The definition of treatment failure included: discontinuing the study for any reason; a total serum bilirubin level greater than or equal to 1.5 mg/dl or increasing to a level equal to or greater than two times the baseline level; and the development of ascites or encephalopathy.
URSO® (ursodiol) tablets are indicated for the treatment of patients with primary biliary cirrhosis.
Hypersensitivity or intolerance to ursodiol or any of the components of the formulation.
Patients with variceal bleeding, hepatic encephalopathy, ascites or in need of an urgent liver transplant, should receive appropriate specific treatment.
Bile acid sequestering agents such as cholestyramine and colestipol may interfere with the action of URSO® by reducing its absorption. Aluminum-based antacids have been shown to adsorb bile acids in vitro and may be expected to interfere with URSO® in the same manner as the bile acid sequestering agents. Estrogens, oral contraceptives, and clofibrate (and perhaps other lipid-lowering drugs) increase hepatic cholesterol secretion, and encourage cholesterol gallstone formation and hence may counteract the effectiveness of URSO® .
In two 24-month oral carcinogenicity studies in mice, ursodiol at doses up to 1,000 mg/kg/day (3,000 mg/m 2 /day) was not tumorigenic. Based on body surface area, for a 50 kg person of average height (1.46 m 2 body surface area), this dose represents 5.4 times the recommended maximum clinical dose of 15 mg/kg/day (555 mg/m 2 /day).
In a two-year oral carcinogenicity study in Fischer 344 rats, ursodiol at doses up to 300 mg/kg/day (1,800 mg/m 2 /day, 3.2 times the recommended maximum human dose based on body surface area) was not tumorigenic.
In a life-span (126-138 weeks) oral carcinogenicity study, Sprague-Dawley rats were treated with doses of 33 to 300 mg/kg/day, 0.4 to 3.2 times the recommended maximum human dose based on body surface area. Ursodiol produced a significantly (p</=0.5, Fisher' exact test) increased incidence of pheochromocytomas of the adrenal medulla in females of the highest dose group.
In 103-week oral carcinogenicity studies of lithocholic acid, a metabolite of ursodiol, doses up to 250 mg/kg/day in mice and 500 mg/kg/day in rats did not produce any tumors. In a 78-week rat study, intrarectal instillation of lithocholic acid (1 mg/kg/day) for 13 months did not produce colorectal tumors. A tumor-promoting effect was observed when it was administered after a single intrarectal dose of a known carcinogen N-methyl-N'-nitro-N-nitrosoguanidine. On the other hand, in a 32-week rat study, ursodiol at a daily dose of 240 mg/kg (1,440 mg/m 2 , 2.6 times the maximum recommended human dose based on body surface area) suppressed the colonic carcinogenic effect of another known carcinogen azoxymethane.
Ursodiol was not genotoxic in the Ames test, the mouse lymphoma cell (L5178Y, TK +/- ) forward mutation test, the human lymphocyte sister chromatid exchange test, the mouse spermatogenia chromosome aberration test, the Chinese hamster micronucleus test and the Chinese hamster bone marrow cell chromosome aberration test.
Ursodiol at oral doses of up to 2,700 mg/kg/day (16,200 mg/m 2 /day, 29 times the recommended maximum human dose based on body surface area) was found to have no effect on fertility and reproductive performance of male and female rats.
Teratology studies have been performed in pregnant rats at oral doses up to 2,000 mg/kg/day (12,000 mg/m 2 /day, 22 times the recommended maximum human dose based on body surface area) and in pregnant rabbits at oral doses up to 300 mg/kg/day (3,600 mg/m 2 /day, 7 times the recommended maximum human dose based on body surface area) and have revealed no evidence of impaired fertility or harm to the fetus due to ursodiol.
There are no adequate or well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
It is not known whether ursodiol is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when URSO® is administered to a nursing mother.
The safety and effectiveness of URSO® in pediatric patients have not been established.
|
||||||||||||||||||||||||||||||||||||||||
Note: Those AEs occurring at the same or higher incidence in the placebo as in the UDCA group have been deleted from this table (this includes diarrhea and thrombocytopenia at 12 months, nausea/vomiting, fever and other toxicity).
UDCA = Ursodeoxycholic acid = Ursodiol
Adverse events are reported regardless of attribution to the test medication.
Accidental or intentional overdosage with ursodiol has not been reported. The most severe manifestation of overdosage would likely consist of diarrhea which should be treated symptomatically.
Single oral doses of ursodiol at 10, 5 and 10 g/kg in mice, rats and dogs, respectively were not lethal. A single oral dose of ursodiol at 1.5 g/kg was lethal in hamsters. Symptoms of acute toxicity were salivation and vomiting in dogs, and ataxia, dyspnea, ptosis, agonal convulsions and coma in hamsters.
The recommended adult dosage for URSO® in the treatment of PBC is 13-15 mg/kg/day administered in four divided doses with food.
Each URSO® film-coated tablet, white, engraved with "URS785", contains 250 mg of ursodiol. Available in bottles of 500 tablets (NDC 58914-785-50) and in bottles of 100's (NDC 58914-785-10). Store at 20°C to 25°C (68°F to 77°F). Dispense in a tight container.
Caution: Federal law prohibits dispensing without a prescription.
Manufactured by:
GLOBAL PHARM INC.
Toronto, Ontario M3B 1Y5
for:
Axcan Scandipharm, Inc.
Birmingham, AL 35242
USA
®Reg. TM of Axcan Pharma US Inc., used under license by Axcan Scandipharm Inc.
April 2000
 |